Rev-dependent indicator T cell line
- PMID: 17627502
- PMCID: PMC2366165
- DOI: 10.2174/157016207781024018
Rev-dependent indicator T cell line
Abstract
Measuring virion infectivity is critical for studying and monitoring the process of HIV-1 infection. The easiest and the most common method utilizes reporter cell lines based on the HIV LTR promoter. The early HIV gene product Tat amplifies expression from the LTR; however, there is a background transcriptional activity that is independent of Tat. Furthermore, LTR activity can be influenced by cellular activation states. We have recently constructed a Rev-dependent expression vector, and as a test of this construct's functionality, we have integrated this vector into a continuous T cell line. This novel indicator cell has no measurable background signal, is not affected by elevated metabolic states, and yet responds robustly to the presence of HIV. The line is able to complete TCID50 assays in 3-5 days, and appears sensitive to both CCR5- and CXCR4-utilizing viruses.
Figures

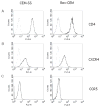
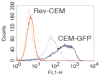


References
-
- Aguilarcordova E, Chinen J, Donehower L, Lewis DE, Belmont JW. A sensitive reporter cell-line for HIV-1 Tat activity, HIV-1 inhibitors, and T-cell activation effects. Aids Research and Human Retroviruses. 1994;10:295–301. - PubMed
-
- Czerniecki BJ, Carter C, Rivoltini L, Koski GK, Kim HI, Weng DE, Roros JG, Hijazi YM, Xu SW, Rosenberg SA, Cohen PA. Calcium ionophore-treated peripheral blood monocytes and dendritic cells rapidly display characteristics of activated dendritic cells. Journal of Immunology. 1997;159:3823–3837. - PubMed
-
- Dorsky DI, Wells M, Harrington RD. Detection of HIV-1 infection with a green fluorescent protein reporter system. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 1996;13:308–313. - PubMed
-
- Felber BK, Pavlakis GN. A quantitative bioassay for HIV-1 based on trans-activation. Science. 1988;239:184–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

