Comparable potency of IFNalpha2 and IFNbeta on immediate JAK/STAT activation but differential down-regulation of IFNAR2
- PMID: 17627610
- PMCID: PMC2267396
- DOI: 10.1042/BJ20070605
Comparable potency of IFNalpha2 and IFNbeta on immediate JAK/STAT activation but differential down-regulation of IFNAR2
Abstract
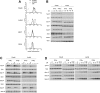
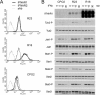
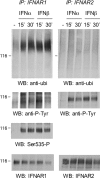
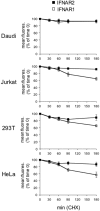
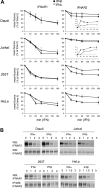
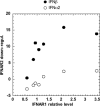
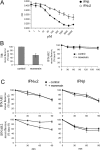
Type I IFNs (interferons) (IFNalpha/beta) form a family of related cytokines that control a variety of cellular functions through binding to a receptor composed of IFNAR (IFNalpha receptor subunit) 1 and 2. Among type I IFNs, the alpha2 and beta subtypes exhibit a large difference in their binding affinities to IFNAR1, and it was suggested that high concentrations of IFNAR1 may compensate for its low intrinsic binding affinity for IFNalpha2. We tested whether receptor-proximal signalling events are sensitive to IFNAR1 surface concentration by investigating the relationship between relative IFNAR1/IFNAR2 surface levels and IFNalpha2 and IFNbeta signalling potencies in several cell lines. For this, we monitored the activation profile of JAK (Janus kinase)/STAT (signal transducer and activator of transcription) proteins, measured basal and ligand-induced surface decay of each receptor subunit and tested the effect of variable IFNAR1 levels on IFNalpha2 signalling potency. Our data show that the cell-surface IFNAR1 level is indeed a limiting factor for assembly of the functional complex, but an increased concentration of it does not translate into an IFNalpha/beta differential JAK/STAT signalling nor does it change the dynamics of the engaged receptor. Importantly, however, our data highlight a differential effect upon routing of IFNAR2. Following binding of IFNalpha2, IFNAR2 is internalized, but, instead of being routed towards degradation as it is when complexed to IFNbeta, it recycles back to the cell surface. These observations suggest strongly that the stability and the intracellular lifetime of the ternary complex account for the differential control of IFNAR2. Moreover, the present study opens up the attractive possibility that endosomal-initiated signalling may contribute to IFNalpha/beta differential bioactivities.
Figures


Similar articles
-
Ligand-induced assembling of the type I interferon receptor on supported lipid bilayers.J Mol Biol. 2004 Jul 30;341(1):303-18. doi: 10.1016/j.jmb.2004.05.059. J Mol Biol. 2004. PMID: 15312780
-
Mutational and structural analysis of the binding interface between type I interferons and their receptor Ifnar2.J Mol Biol. 1999 Nov 19;294(1):223-37. doi: 10.1006/jmbi.1999.3230. J Mol Biol. 1999. PMID: 10556041
-
Initial expression of interferon alpha receptor 2 (IFNAR2) on CD34-positive cells and its down-regulation correlate with clinical response to interferon therapy in chronic myelogenous leukemia.Eur J Haematol. 2004 Sep;73(3):191-205. doi: 10.1111/j.1600-0609.2004.00275.x. Eur J Haematol. 2004. PMID: 15287917
-
Insulin-like growth factor-I receptor signal transduction and the Janus Kinase/Signal Transducer and Activator of Transcription (JAK-STAT) pathway.Biofactors. 2009 Jan-Feb;35(1):76-81. doi: 10.1002/biof.20. Biofactors. 2009. PMID: 19319849 Review.
-
Systems biology of JAK/STAT signalling.Essays Biochem. 2008;45:109-20. doi: 10.1042/BSE0450109. Essays Biochem. 2008. PMID: 18793127 Review.
Cited by
-
Transient oscillatory dynamics of interferon beta signaling in macrophages.BMC Syst Biol. 2013 Jul 9;7:59. doi: 10.1186/1752-0509-7-59. BMC Syst Biol. 2013. PMID: 23837526 Free PMC article.
-
Basal Activation of Type I Interferons (Alpha2 and Beta) and 2'5'OAS Genes: Insights into Differential Expression Profiles of Interferon System Components in Systemic Sclerosis.Int J Rheumatol. 2011;2011:275617. doi: 10.1155/2011/275617. Epub 2011 Nov 1. Int J Rheumatol. 2011. PMID: 22121373 Free PMC article.
-
Molecular and cellular factors determining the functional pleiotropy of cytokines.FEBS J. 2023 May;290(10):2525-2552. doi: 10.1111/febs.16420. Epub 2022 Mar 14. FEBS J. 2023. PMID: 35246947 Free PMC article. Review.
-
Respiratory syncytial virus interferon antagonist NS1 protein suppresses and skews the human T lymphocyte response.PLoS Pathog. 2011 Apr;7(4):e1001336. doi: 10.1371/journal.ppat.1001336. Epub 2011 Apr 21. PLoS Pathog. 2011. PMID: 21533073 Free PMC article.
-
Interferon-α induces neurotoxicity through activation of the type I receptor and the GluN2A subunit of the NMDA receptor.J Interferon Cytokine Res. 2015 Apr;35(4):317-24. doi: 10.1089/jir.2014.0105. Epub 2014 Dec 17. J Interferon Cytokine Res. 2015. PMID: 25517826 Free PMC article.
References
-
- Vilcek J. Fifty years of interferon research: aiming at a moving target. Immunity. 2006;25:343–348. - PubMed
-
- Pestka S., Krause C. D., Walter M. R. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 2004;202:8–32. - PubMed
-
- Haan C., Kreis S., Margue C., Behrmann I. Jaks and cytokine receptors: an intimate relationship. Biochem. Pharmacol. 2006;72:1538–1546. - PubMed
-
- Rani M. R., Ransohoff R. M. Alternative and accessory pathways in the regulation of IFN-β-mediated gene expression. J. Interferon Cytokine Res. 2005;25:788–798. - PubMed

