Characterization of the potent 5-HT(1A/B) receptor antagonist and serotonin reuptake inhibitor SB-649915: preclinical evidence for hastened onset of antidepressant/anxiolytic efficacy
- PMID: 17627673
- PMCID: PMC6726354
- DOI: 10.1111/j.1527-3458.2007.00012.x
Characterization of the potent 5-HT(1A/B) receptor antagonist and serotonin reuptake inhibitor SB-649915: preclinical evidence for hastened onset of antidepressant/anxiolytic efficacy
Abstract
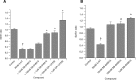
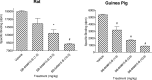
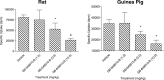
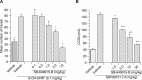
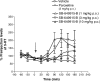
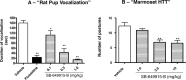
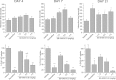
An increase in brain serotonin (5-HT) levels is thought to be a key mechanism of action responsible for generating antidepressant efficacy. It has been proven that selective serotonin reuptake inhibitors are effective antidepressants, but the delay to therapeutic onset of these agents is thought to be due to the time required for 5-HT1A, and possibly 5-HT1B, autoreceptors to desensitize. Therefore, an agent incorporating 5-HT reuptake inhibition coupled with 5-HT1A and/or 5-HT1B autoreceptor antagonism may provide a fast-acting clinical agent. The current studies review the profile of SB-649915 (6-[(1-{2-[(2-methylquinolin-5-yl)oxy]ethyl}piperidin-4-yl)methyl]-2H-1,4-benzoxazin-3(4H)-one), a novel compound with high affinity for human (h) 5-HT1A and 5-HT1B receptors (pKi values of 8.6 and 8.0, respectively) as well as the (h) 5-HT transporter (SERT) (pKi value of 9.3). SB-649915 behaved as an antagonist at both 5-HT1A and 5-HT1B receptors in vitro and in vivo, reversing 5-HT, (+)8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT) and SKF99101-induced functional/behavioral responses. Furthermore, it inhibited [3H]5-HT reuptake in rat cortical synaptosomes, in vitro and ex vivo. In electrophysiological studies SB-649915 had no effect on rat dorsal raphe neuronal cell firing per se, but reversed 8-OH-DPAT-induced inhibition of firing both in vitro and in vivo. In addition, in a microdialysis study, it produced an acute increase in extracellular 5-HT in forebrain structures of the rat. Finally, SB-649915 demonstrated acute anxiolytic activity in both rodent and non-human primate and reduced the latency to onset of anxiolytic behavior, compared to paroxetine, in the rat social interaction paradigm. In summary, SB-649915 is a novel, potent 5-HT1A/1B autoreceptor antagonist, and 5-HT reuptake inhibitor. This particular pharmacological profile provides a novel mechanism that could offer fast-acting antidepressant activity.
Figures


Similar articles
-
Dual- and triple-acting agents for treating core and co-morbid symptoms of major depression: novel concepts, new drugs.Neurotherapeutics. 2009 Jan;6(1):53-77. doi: 10.1016/j.nurt.2008.10.039. Neurotherapeutics. 2009. PMID: 19110199 Free PMC article. Review.
-
SB-649915, a novel, potent 5-HT1A and 5-HT1B autoreceptor antagonist and 5-HT re-uptake inhibitor in native tissue.Eur J Pharmacol. 2006 Apr 24;536(1-2):54-61. doi: 10.1016/j.ejphar.2006.02.042. Epub 2006 Feb 28. Eur J Pharmacol. 2006. PMID: 16571351
-
Simultaneous blockade of 5-HT1A/B receptors and 5-HT transporters results in acute increases in extracellular 5-HT in both rats and guinea pigs: in vivo characterization of the novel 5-HT1A/B receptor antagonist/5-HT transport inhibitor SB-649915-B.Psychopharmacology (Berl). 2007 May;192(1):121-33. doi: 10.1007/s00213-006-0691-x. Epub 2007 Jan 30. Psychopharmacology (Berl). 2007. PMID: 17265079
-
SB-649915-B, a novel 5-HT1A/B autoreceptor antagonist and serotonin reuptake inhibitor, is anxiolytic and displays fast onset activity in the rat high light social interaction test.Neuropsychopharmacology. 2007 Oct;32(10):2163-72. doi: 10.1038/sj.npp.1301341. Epub 2007 Mar 14. Neuropsychopharmacology. 2007. PMID: 17356576
-
SB-236057-A: a selective 5-HT1B receptor inverse agonist.CNS Drug Rev. 2001 Winter;7(4):433-44. doi: 10.1111/j.1527-3458.2001.tb00209.x. CNS Drug Rev. 2001. PMID: 11830759 Free PMC article. Review.
Cited by
-
Dual- and triple-acting agents for treating core and co-morbid symptoms of major depression: novel concepts, new drugs.Neurotherapeutics. 2009 Jan;6(1):53-77. doi: 10.1016/j.nurt.2008.10.039. Neurotherapeutics. 2009. PMID: 19110199 Free PMC article. Review.
-
Preclinical characterization of WAY-211612: a dual 5-HT uptake inhibitor and 5-HT (1A) receptor antagonist and potential novel antidepressant.Br J Pharmacol. 2009 May;157(2):307-19. doi: 10.1111/j.1476-5381.2009.00146.x. Epub 2009 Mar 26. Br J Pharmacol. 2009. PMID: 19338583 Free PMC article.
-
Neuroimaging of reward mechanisms in Gambling disorder: an integrative review.Mol Psychiatry. 2019 May;24(5):674-693. doi: 10.1038/s41380-018-0230-2. Epub 2018 Sep 13. Mol Psychiatry. 2019. PMID: 30214041 Review.
-
Exercise benefits brain function: the monoamine connection.Brain Sci. 2013 Jan 11;3(1):39-53. doi: 10.3390/brainsci3010039. Brain Sci. 2013. PMID: 24961306 Free PMC article.
-
Dorsal Raphe Dual Serotonin-Glutamate Neurons Drive Reward by Establishing Excitatory Synapses on VTA Mesoaccumbens Dopamine Neurons.Cell Rep. 2019 Jan 29;26(5):1128-1142.e7. doi: 10.1016/j.celrep.2019.01.014. Cell Rep. 2019. PMID: 30699344 Free PMC article.
References
-
- Adham N, Romanienko P, Hartig P, Weinshank RL, Brancheck TA (1992) The rat 5‐hydroxytryptamine1B receptor is the species homologue of the human 5‐HT1D β receptor. Mol Pharmacol 41:1‐7. - PubMed
-
- Artigas F, Celada P, Laruelle M, Adell A (2001) How does pindolol improve antidepressant action? TIPS 22:224‐228. - PubMed
-
- Artigas F, Perez V, Alvarez E (1994) Pindolol induces a rapid improvement of depressed patients treated with serotonin reuptake inhibitors. Arch Gen Psychiatry 51:48‐51. - PubMed
-
- Artigas F, Romero L, De Montigny C, Blier P (1996) Acceleration of the effect of selected antidepressant drugs in major depression by 5‐HT1A antagonists. TINS 19:378‐383. - PubMed
-
- Atkinson PJ, Bromidge SM, Duxon MS, Gaster LM, Hadley MS, Hammond B, Johnson CN, Middlemiss DN, North SE, Price GW, et al. (2005) 3,4‐Dihydro‐2H‐benzoxazinones are 5‐HT1A receptor antagonists with potent 5‐HT reuptake inhibitory activity. Bioorg Med Chem Lett 15:737‐741. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

