Morphometric analysis of the C57BL/6J mouse brain
- PMID: 17627846
- PMCID: PMC2176152
- DOI: 10.1016/j.neuroimage.2007.05.046
Morphometric analysis of the C57BL/6J mouse brain
Abstract
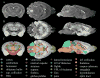
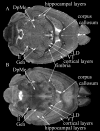
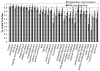
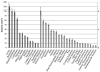
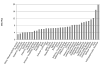
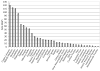
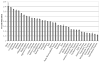
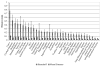
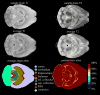
Magnetic resonance microscopy (MRM), when used in conjunction with active staining, can produce high-resolution, high-contrast images of the mouse brain. Using MRM, we imaged in situ the fixed, actively stained brains of C57BL/6J mice in order to characterize the neuroanatomical phenotype and produce a digital atlas. The brains were scanned within the cranium vault to preserve the brain morphology, avoid distortions, and to allow an unbiased shape analysis. The high-resolution imaging used a T1-weighted scan at 21.5 microm isotropic resolution, and an eight-echo multi-echo scan, post-processed to obtain an enhanced T2 image at 43 microm resolution. The two image sets were used to segment the brain into 33 anatomical structures. Volume, area, and shape characteristics were extracted for all segmented brain structures. We also analyzed the variability of volumes, areas, and shape characteristics. The coefficient of variation of volume had an average value of 7.0%. Average anatomical images of the brain for both the T1-weighted and T2 images were generated, together with an average shape atlas, and a probabilistic atlas for 33 major structures. These atlases, with their associated meta-data, will serve as baseline for identifying neuroanatomical phenotypes of additional strains, and mouse models now under study. Our efforts were directed toward creating a baseline for comparison with other mouse strains and models of neurodegenerative diseases.
Figures

References
-
- Ali AA, Dale AM, et al. Automated segmentation of neuroanatomical structures in multispectral MR microscopy of the mouse brain. NeuroImage. 2005;27(2):425–435. - PubMed
-
- Ashburner J. Computational Neuroanatomy. London: University College; 2000.
-
- Badea A, Kostopoulos GK, et al. Surface visualization of electromagnetic brain activity. J Neurosci Methods. 2003;127(2):137–47. - PubMed
-
- Barabasi AL, Stanley HE. Fractal Concepts in Surface Growth. Cambridge: Cambridge University Press; 1995.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

