Bladder afferent sensitivity in wild-type and TRPV1 knockout mice
- PMID: 17627983
- PMCID: PMC2277033
- DOI: 10.1113/jphysiol.2007.139147
Bladder afferent sensitivity in wild-type and TRPV1 knockout mice
Abstract
Understanding bladder afferent pathways may reveal novel targets for therapy of lower urinary tract disorders such as overactive bladder syndrome and cystitis. Several potential candidate molecules have been postulated as playing a significant role in bladder function. One such candidate is the transient receptor potential vanilloid 1 (TRPV1) ion channel. Mice lacking the TRPV1 channel have altered micturition thresholds suggesting that TRPV1 channels may play a role in the detection of bladder filling. The aim of this study was therefore to investigate the role of TRPV1 receptors in controlling bladder afferent sensitivity in the mouse using pharmacological receptor blockade and genetic deletion of the channel. Multiunit afferent activity was recorded in vitro from bladder afferents taken from wild-type (TRPV+/+) mice and knockout (TRPV1-/-) mice. In wild-type preparations, ramp distension of the bladder to a maximal pressure of 40 mmHg produced a graded increase in afferent activity. Bath application of the TRPV1 antagonist capsazepine (10 mum) caused a significant attenuation of afferent discharge in TRPV1+/+ mice. Afferent responses to distension were significantly attenuated in TRPV1-/- mice in which sensitivity to intravesical hydrochloric acid (50 mm) and capsaicin (10 microm) were also blunted. Altered mechanosensitivity occurred in the absence of any changes in the pressure-volume relationship during filling indicating that this was not secondary to a change in bladder compliance. Single-unit analysis was used to classify individual afferents into low-threshold and high-threshold fibres. Low threshold afferent responses were attenuated in TRPV1-/- mice compared to the TRPV1+/+ littermates while surprisingly high threshold afferent sensitivity was unchanged. While TRPV1 channels are not considered to be mechanically gated, the present study demonstrates a clear role for TRPV1 in the excitability of particularly low threshold bladder afferents. This suggests that TRPV1 may play an important role in normal bladder function.
Figures





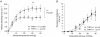
Similar articles
-
Jejunal afferent nerve sensitivity in wild-type and TRPV1 knockout mice.J Physiol. 2004 Nov 1;560(Pt 3):867-81. doi: 10.1113/jphysiol.2004.071746. Epub 2004 Aug 26. J Physiol. 2004. PMID: 15331673 Free PMC article.
-
Functional roles of TRPV1 channels in lower urinary tract irritated by acetic acid: in vivo evaluations of the sex difference in decerebrate unanesthetized mice.Am J Physiol Renal Physiol. 2010 Jun;298(6):F1351-9. doi: 10.1152/ajprenal.00695.2009. Epub 2010 Mar 17. Am J Physiol Renal Physiol. 2010. PMID: 20237234
-
Differential effects of ASIC3 and TRPV1 deletion on gastroesophageal sensation in mice.Am J Physiol Gastrointest Liver Physiol. 2008 Jan;294(1):G130-8. doi: 10.1152/ajpgi.00388.2007. Epub 2007 Nov 1. Am J Physiol Gastrointest Liver Physiol. 2008. PMID: 17975130
-
TRPV1: a therapeutic target for novel analgesic drugs?Trends Mol Med. 2006 Nov;12(11):545-54. doi: 10.1016/j.molmed.2006.09.001. Epub 2006 Sep 25. Trends Mol Med. 2006. PMID: 16996800 Review.
-
Ion channel and receptor mechanisms of bladder afferent nerve sensitivity.Auton Neurosci. 2010 Feb 16;153(1-2):26-32. doi: 10.1016/j.autneu.2009.07.003. Epub 2009 Jul 25. Auton Neurosci. 2010. PMID: 19632906 Review.
Cited by
-
Activation of transient receptor potential vanilloid-1 (TRPV1) influences how retinal ganglion cell neurons respond to pressure-related stress.Channels (Austin). 2015;9(2):102-13. doi: 10.1080/19336950.2015.1009272. Channels (Austin). 2015. PMID: 25713995 Free PMC article.
-
Spinal Reflex Control of Arterial Blood Pressure: The Role of TRP Channels and Their Endogenous Eicosanoid Modulators.Front Physiol. 2022 Feb 23;13:838175. doi: 10.3389/fphys.2022.838175. eCollection 2022. Front Physiol. 2022. PMID: 35283783 Free PMC article. Review.
-
Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents.J Neurophysiol. 2008 Jan;99(1):244-53. doi: 10.1152/jn.01049.2007. Epub 2007 Nov 14. J Neurophysiol. 2008. PMID: 18003875 Free PMC article.
-
An exploration of the control of micturition using a novel in situ arterially perfused rat preparation.Front Neurosci. 2011 May 13;5:62. doi: 10.3389/fnins.2011.00062. eCollection 2011. Front Neurosci. 2011. PMID: 21625609 Free PMC article.
-
Absence of transient receptor potential vanilloid-1 accelerates stress-induced axonopathy in the optic projection.J Neurosci. 2014 Feb 26;34(9):3161-70. doi: 10.1523/JNEUROSCI.4089-13.2014. J Neurosci. 2014. PMID: 24573275 Free PMC article.
References
-
- Avelino A, Cruz F. TRPV1 (vanilloid receptor) in the urinary tract: expression, function and clinical applications. Naunyn Schmiedebergs Arch Pharmacol. 2006;373:287–299. - PubMed
-
- Avelino A, Cruz C, Nagy I, Cruz F. Vanilloid receptor 1 expression in the rat urinary tract. Neuroscience. 2002;109:787–798. - PubMed
-
- Bahns E, Halsband U, Janig W. Responses of sacral visceral afferents from the lower urinary tract, colon and anus to mechanical stimulation. Pflugers Arch. 1987;410:296–303. - PubMed
-
- Birder LA. Involvement of the urinary bladder urothelium in signaling in the lower urinary tract. Proc West Pharmacol Soc. 2001;44:85–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

