Genetic deletion of SK2 channels in mouse inner hair cells prevents the developmental linearization in the Ca2+ dependence of exocytosis
- PMID: 17627990
- PMCID: PMC2096744
- DOI: 10.1113/jphysiol.2007.136630
Genetic deletion of SK2 channels in mouse inner hair cells prevents the developmental linearization in the Ca2+ dependence of exocytosis
Abstract
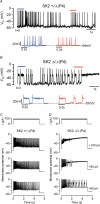
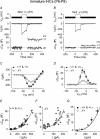
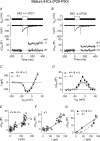
Inner hair cells (IHCs), the primary sensory receptors of the mammalian cochlea, fire spontaneous Ca(2+) action potentials (APs) only before the onset of hearing. Although a role for APs in the developing auditory system has not been determined it could, by analogy with other sensory systems, guide the functional maturation of the cochlea before experience-driven activity begins. Spontaneous APs in immature IHCs are shaped by a variety of ion channels including that of the small conductance Ca(2+)-activated K(+) current (SK2), which is only transiently expressed in immature cells. Using SK2 knockout mice we found that SK2 channels are not required for generating APs but are essential for sustaining continuous repetitive spontaneous AP activity in pre-hearing IHCs. Therefore we used this mutant mouse as a model to study possible developmental implications of disrupted AP activity. Immature mutant IHCs showed impaired exocytotic responses, which are likely to be due to the expression of fewer Ca(2+) channels. Exocytosis was also impaired in adult mutant IHCs, although in this case it resulted from a reduced Ca(2+) efficiency and increased Ca(2+) dependence of the synaptic machinery. Since SK2 channels can only have a functional influence on IHCs during immature development and are not directly involved in neurotransmitter release, the altered Ca(2+) dependence of exocytosis in adult IHCs is likely to be a consequence of their disrupted AP activity at immature stages.
Figures






Similar articles
-
Calcium-Induced calcium release during action potential firing in developing inner hair cells.Biophys J. 2015 Mar 10;108(5):1003-12. doi: 10.1016/j.bpj.2014.11.3489. Biophys J. 2015. PMID: 25762313 Free PMC article.
-
CaV1.3 channels are essential for development and presynaptic activity of cochlear inner hair cells.J Neurosci. 2003 Nov 26;23(34):10832-40. doi: 10.1523/JNEUROSCI.23-34-10832.2003. J Neurosci. 2003. PMID: 14645476 Free PMC article.
-
Expression of the SK2 calcium-activated potassium channel is required for cholinergic function in mouse cochlear hair cells.J Physiol. 2008 Nov 15;586(22):5471-85. doi: 10.1113/jphysiol.2008.160077. Epub 2008 Sep 25. J Physiol. 2008. PMID: 18818242 Free PMC article.
-
How to build an inner hair cell: challenges for regeneration.Hear Res. 2007 May;227(1-2):3-10. doi: 10.1016/j.heares.2006.12.005. Epub 2006 Dec 16. Hear Res. 2007. PMID: 17258412 Review.
-
Spontaneous activity in the developing auditory system.Cell Tissue Res. 2015 Jul;361(1):65-75. doi: 10.1007/s00441-014-2007-5. Epub 2014 Oct 9. Cell Tissue Res. 2015. PMID: 25296716 Free PMC article. Review.
Cited by
-
Functional maturation of the exocytotic machinery at gerbil hair cell ribbon synapses.J Physiol. 2009 Apr 15;587(Pt 8):1715-26. doi: 10.1113/jphysiol.2009.168542. Epub 2009 Feb 23. J Physiol. 2009. PMID: 19237422 Free PMC article.
-
The Coupling between Ca2+ Channels and the Exocytotic Ca2+ Sensor at Hair Cell Ribbon Synapses Varies Tonotopically along the Mature Cochlea.J Neurosci. 2017 Mar 1;37(9):2471-2484. doi: 10.1523/JNEUROSCI.2867-16.2017. Epub 2017 Feb 2. J Neurosci. 2017. PMID: 28154149 Free PMC article.
-
Calcium-Induced calcium release during action potential firing in developing inner hair cells.Biophys J. 2015 Mar 10;108(5):1003-12. doi: 10.1016/j.bpj.2014.11.3489. Biophys J. 2015. PMID: 25762313 Free PMC article.
-
Alternative splice isoforms of small conductance calcium-activated SK2 channels differ in molecular interactions and surface levels.Channels (Austin). 2014;8(1):62-75. doi: 10.4161/chan.27470. Epub 2014 Jan 6. Channels (Austin). 2014. PMID: 24394769 Free PMC article.
-
Presynaptic maturation in auditory hair cells requires a critical period of sensory-independent spiking activity.Proc Natl Acad Sci U S A. 2013 May 21;110(21):8720-5. doi: 10.1073/pnas.1219578110. Epub 2013 May 6. Proc Natl Acad Sci U S A. 2013. PMID: 23650376 Free PMC article.
References
-
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

