Functional groups of ryanodine receptors in rat ventricular cells
- PMID: 17627991
- PMCID: PMC2277248
- DOI: 10.1113/jphysiol.2007.136549
Functional groups of ryanodine receptors in rat ventricular cells
Abstract
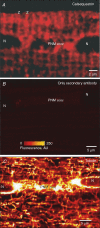
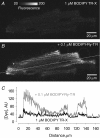
Ryanodine receptors (RyR2s) are ion channels in the sarcoplasmic reticulum (SR) that are responsible for Ca2+ release in rat ventricular myocytes. Localization of RyR2s is therefore crucial for our understanding of contraction and other Ca2+-dependent intracellular processes. Recent results (e.g. circular waves and Ca2+ sparks in perinuclear area) raised questions about the classical views of RyR2 distribution and organization within ventricular cells. A Ca2+ spark is a fluorescent signal reflecting the activation of a small group of RyR2s. Frequency and spatio-temporal characteristics of Ca2+ sparks depend on the state of cytoplasmic and intraluminal macromolecular complexes regulating cardiac RyR2 function. We employed electron microscopy, confocal imaging of spontaneous Ca2+ sparks and immunofluorescence to visualize the distribution of RyR2s in ventricular myocytes and to evaluate the local involvement of the macromolecular complexes in regulation of functional activity of the RyR2 group. An electron microscopy study revealed that the axial tubules of the transverse-axial tubular system probably do not have junctions with the network SR (nSR). The nSR was found to be wrapped around intermyofibrillar mitochondria and contained structures similar to feet of the junctional cleft. Treatment of ventricular myocytes with antibodies against RyR2 showed that in addition to the junctional SR, a small number of RyR2s can be localized at the middle of the sarcomere and in the zone of perinuclear mitochondria. Recordings of spontaneous Ca2+ sparks showed the existence of functional groups of RyR2s in these intracellular compartments. We found that within the sarcomere about 20% of Ca2+ sparks were not colocalized with the zone of the junctional or corbular SR (Z-line zone). The spatio-temporal characteristics of sparks found in the Z-line and A-band zones were very similar, whereas sparks from the zone of the perinuclear mitochondria were about 25% longer. Analysis of the initiation sites of Ca2+ sparks within the same junctional SR cluster suggested that 18-25 RyR2s are in the functional group producing a spark. Because of the similarity of the spatio-temporal characteristics of sarcomeric sparks and ultrastructural characteristics of nSR, we suggest that the functional groups of RyR2s in the middle of the sarcomere are macromolecular complexes of approximately 20 RyR2s with regulatory proteins. Our data allowed us to conclude that a significant number of functional RyR2s is located in the middle of the sarcomere and in the zone of perinuclear mitochondria. These RyR2s could contribute to excitation-contraction coupling, mitochondrial and nuclear signalling, and Ca2+-dependent gene regulation, but their existence raises many additional questions.
Figures








Similar articles
-
Regional differences in spontaneous Ca2+ spark activity and regulation in cat atrial myocytes.J Physiol. 2006 May 1;572(Pt 3):799-809. doi: 10.1113/jphysiol.2005.103267. J Physiol. 2006. PMID: 16484302 Free PMC article.
-
Triadin overexpression stimulates excitation-contraction coupling and increases predisposition to cellular arrhythmia in cardiac myocytes.Circ Res. 2005 Apr 1;96(6):651-8. doi: 10.1161/01.RES.0000160609.98948.25. Epub 2005 Feb 24. Circ Res. 2005. PMID: 15731460
-
Protein protein interactions between triadin and calsequestrin are involved in modulation of sarcoplasmic reticulum calcium release in cardiac myocytes.J Physiol. 2007 Aug 15;583(Pt 1):71-80. doi: 10.1113/jphysiol.2007.136879. Epub 2007 Jun 14. J Physiol. 2007. PMID: 17569730 Free PMC article.
-
Excitation-contraction coupling in heart: new insights from Ca2+ sparks.Cell Calcium. 1996 Aug;20(2):129-40. doi: 10.1016/s0143-4160(96)90102-5. Cell Calcium. 1996. PMID: 8889204 Review.
-
Imaging microdomain Ca2+ in muscle cells.Circ Res. 2004 Apr 30;94(8):1011-22. doi: 10.1161/01.RES.0000125883.68447.A1. Circ Res. 2004. PMID: 15117829 Review.
Cited by
-
Modelling cardiac calcium sparks in a three-dimensional reconstruction of a calcium release unit.J Physiol. 2012 Sep 15;590(18):4403-22. doi: 10.1113/jphysiol.2012.227926. Epub 2012 Apr 10. J Physiol. 2012. PMID: 22495592 Free PMC article.
-
Characteristics and possible functions of mitochondrial Ca(2+) transport mechanisms.Biochim Biophys Acta. 2009 Nov;1787(11):1291-308. doi: 10.1016/j.bbabio.2008.12.011. Epub 2009 Jan 6. Biochim Biophys Acta. 2009. PMID: 19161975 Free PMC article. Review.
-
Subcellular heterogeneity of ryanodine receptor properties in ventricular myocytes with low T-tubule density.PLoS One. 2011;6(10):e25100. doi: 10.1371/journal.pone.0025100. Epub 2011 Oct 13. PLoS One. 2011. PMID: 22022376 Free PMC article.
-
Nanospaces between endoplasmic reticulum and mitochondria as control centres of pancreatic β-cell metabolism and survival.Protoplasma. 2012 Feb;249 Suppl 1:S49-58. doi: 10.1007/s00709-011-0349-3. Epub 2011 Nov 22. Protoplasma. 2012. PMID: 22105567 Review.
-
Ontogeny of cardiomyocytes: ultrastructure optimization to meet the demand for tight communication in excitation-contraction coupling and energy transfer.Philos Trans R Soc Lond B Biol Sci. 2022 Nov 21;377(1864):20210321. doi: 10.1098/rstb.2021.0321. Epub 2022 Oct 3. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 36189816 Free PMC article. Review.
References
-
- Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2001.
-
- Bers DM. Macromolecular complexes regulating cardiac ryanodine receptor function. J Mol Cell Cardiol. 2004;37:417–429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

