HIF-1alpha and HIF-2alpha play a central role in stretch-induced but not shear-stress-induced angiogenesis in rat skeletal muscle
- PMID: 17627993
- PMCID: PMC2277012
- DOI: 10.1113/jphysiol.2007.136325
HIF-1alpha and HIF-2alpha play a central role in stretch-induced but not shear-stress-induced angiogenesis in rat skeletal muscle
Abstract
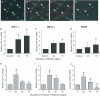
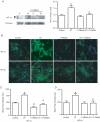
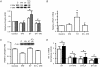
Angiogenesis, which is essential for the physiological adaptation of skeletal muscle to exercise, occurs in response to the mechanical forces of elevated capillary shear stress and cell stretch. Increased production of VEGF is a characteristic of endothelial cells undergoing either stretch- or shear-stress-induced angiogenesis. Because VEGF production is regulated by hypoxia inducible factors (HIFs), we examined whether HIFs play a significant role in the angiogenic process initiated by these mechanical forces. Rat extensor digitorum longus (EDL) muscles were overloaded to induce stretch, or exposed to the dilator prazosin to elevate capillary shear stress, and capillaries from these muscles were isolated by laser capture microdissection for RNA analysis. HIF-1alpha and HIF-2alpha transcript levels increased after 4 and 7 days of stretch, whereas a transient early induction of HIF-1alpha and HIF-2alpha transcripts was detected in capillaries from prazosin-treated muscles. Skeletal muscle microvascular endothelial cells exposed to 10% stretch in vitro showed an elevation in HIF-1alpha and HIF-2alpha mRNA, which was preceded by increases in HIF-binding activity. Conversely, HIF-1alpha and HIF-2alpha mRNA were reduced significantly, and HIF-alpha proteins were undetectable, after 24 h exposure to elevated shear stress (16 dyn cm(-2) (16 x10(-5) N cm(-2)). Given the disparate regulation of HIFs in response to these mechanical stimuli, we tested the requirement of HIF-alpha proteins in stretch- and shear-stress-induced angiogenesis by impeding HIF accumulation through use of the geldanamycin derivative 17-DMAG. Treatment with 17-DMAG significantly impaired stretch-induced, but not shear-stress-induced, angiogenesis. Together, these results illustrate that activation of HIF-1alpha and HIF-2alpha contributes significantly to stretch- but not to shear-stress-induced capillary growth.
Figures
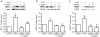
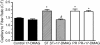
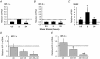
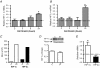
Similar articles
-
Constitutive expression of HIF-1alpha and HIF-2alpha in bone marrow stromal cells differentially promotes their proangiogenic properties.Stem Cells. 2008 Oct;26(10):2634-43. doi: 10.1634/stemcells.2008-0369. Epub 2008 Aug 7. Stem Cells. 2008. PMID: 18687993
-
Activation of fractalkine/CX3CR1 by vascular endothelial cells induces angiogenesis through VEGF-A/KDR and reverses hindlimb ischaemia.Cardiovasc Res. 2008 May 1;78(2):333-40. doi: 10.1093/cvr/cvm067. Epub 2007 Nov 11. Cardiovasc Res. 2008. PMID: 18006432
-
Association between shear stress, angiogenesis, and VEGF in skeletal muscles in vivo.Microcirculation. 2001 Aug;8(4):229-41. doi: 10.1038/sj/mn/7800074. Microcirculation. 2001. PMID: 11528531
-
Adaptation of skeletal muscle microvasculature to increased or decreased blood flow: role of shear stress, nitric oxide and vascular endothelial growth factor.J Vasc Res. 2009;46(5):504-12. doi: 10.1159/000226127. Epub 2009 Jun 26. J Vasc Res. 2009. PMID: 19556804 Review.
-
In vivo shear stress response.Biochem Soc Trans. 2011 Dec;39(6):1633-8. doi: 10.1042/BST20110715. Biochem Soc Trans. 2011. PMID: 22103499 Review.
Cited by
-
Angiogenesis is required for stress fracture healing in rats.Bone. 2013 Jan;52(1):212-9. doi: 10.1016/j.bone.2012.09.035. Epub 2012 Oct 5. Bone. 2013. PMID: 23044046 Free PMC article.
-
Molecular mechanisms of hypoxia-inducible factor-induced pulmonary arterial smooth muscle cell alterations in pulmonary hypertension.J Physiol. 2016 Mar 1;594(5):1167-77. doi: 10.1113/JP270689. Epub 2015 Sep 30. J Physiol. 2016. PMID: 26228924 Free PMC article. Review.
-
Multifaceted Interplay between Hormones, Growth Factors and Hypoxia in the Tumor Microenvironment.Cancers (Basel). 2022 Jan 21;14(3):539. doi: 10.3390/cancers14030539. Cancers (Basel). 2022. PMID: 35158804 Free PMC article. Review.
-
Hypoxia-inducible factor-1α protein negatively regulates load-induced bone formation.J Biol Chem. 2011 Dec 30;286(52):44449-56. doi: 10.1074/jbc.M111.276683. Epub 2011 Nov 12. J Biol Chem. 2011. PMID: 22081627 Free PMC article.
-
Angiotensin II evokes angiogenic signals within skeletal muscle through co-ordinated effects on skeletal myocytes and endothelial cells.PLoS One. 2014 Jan 9;9(1):e85537. doi: 10.1371/journal.pone.0085537. eCollection 2014. PLoS One. 2014. PMID: 24416421 Free PMC article.
References
-
- Armstrong RB, Ianuzzo CD, Laughlin MH. Blood flow and glycogen use in hypertrophied rat muscles during exercise. J Appl Physiol. 1986;61:683–687. - PubMed
-
- Ausprunk DH, Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1977;14:53–65. - PubMed
-
- Berra E, Milanini J, Richard DE, Le Gall M, Vinals F, Gothie E, Roux D, Pages G, Pouyssegur J. Signaling angiogenesis via p42/p44 MAP kinase and hypoxia. Biochem Pharmacol. 2000;60:1171–1178. - PubMed
-
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nature Cell Biol. 2001;3:1014–1019. - PubMed
-
- Boyd PJ, Doyle J, Gee E, Pallan S, Haas TL. Mitogen-activated protein kinase signaling regulates endothelial cell assembly into networks and the expression of MT1-MMP and MMP—. Am J Physiol Cell Physiol. 2005;288:C659–C668. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

