Molecular imaging of bone marrow mononuclear cell homing and engraftment in ischemic myocardium
- PMID: 17628019
- PMCID: PMC3657503
- DOI: 10.1634/stemcells.2007-0041
Molecular imaging of bone marrow mononuclear cell homing and engraftment in ischemic myocardium
Abstract
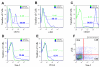
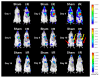
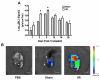
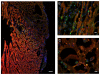
Bone marrow mononuclear cell (BMMC) therapy shows promise as a treatment for ischemic heart disease. However, the ability to monitor long-term cell fate remains limited. We hypothesized that molecular imaging could be used to track stem cell homing and survival after myocardial ischemia-reperfusion (I/R) injury. We first harvested donor BMMCs from adult male L2G85 transgenic mice constitutively expressing both firefly luciferase (Fluc) and enhanced green fluorescence protein reporter gene. Fluorescence-activated cell sorting analysis revealed approximately 0.07% of the population to consist of classic hematopoietic stem cells (lin-, thy-int, c-kit+, Sca-1+). Afterward, adult female FVB recipients (n = 38) were randomized to sham surgery or acute I/R injury. Animals in the sham (n = 16) and I/R (n = 22) groups received 5 x 10(6) of the L2G85-derived BMMCs via tail vein injection. Bioluminescence imaging (BLI) was used to track cell migration and survival in vivo for 4 weeks. BLI showed preferential homing of BMMCs to hearts with I/R injury compared with sham hearts within the first week following cell injection. Ex vivo analysis of explanted hearts by histology confirmed BLI imaging results, and quantitative real-time polymerase chain reaction (for the male Sry gene) further demonstrated a greater number of BMMCs in hearts with I/R injury compared with the sham group. Functional evaluation by echocardiography demonstrated a trend toward improved left ventricular fractional shortening in animals receiving BMMCs. Taken together, these data demonstrate that molecular imaging can be used to successfully track BMMC therapy in murine models of heart disease. Specifically, we have demonstrated that systemically delivered BMMCs preferentially home to and are retained by injured myocardium. Disclosure of potential conflicts of interest is found at the end of this article.
Figures


References
-
- Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001 Apr 5;410(6829):701–705. - PubMed
-
- Perin EC, Dohmann HF, Borojevic R, et al. Improved exercise capacity and ischemia 6 and 12 months after transendocardial injection of autologous bone marrow mononuclear cells for ischemic cardiomyopathy. Circulation. 2004 Sep 14;110(11 Suppl 1):II213–218. - PubMed
-
- Strauer BE, Brehm M, Zeus T, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002 Oct 8;106(15):1913–1918. - PubMed
-
- Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004 Jul 10;364(9429):141–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

