Glioma selectivity of magnetically targeted nanoparticles: a role of abnormal tumor hydrodynamics
- PMID: 17628157
- PMCID: PMC2094531
- DOI: 10.1016/j.jconrel.2007.05.030
Glioma selectivity of magnetically targeted nanoparticles: a role of abnormal tumor hydrodynamics
Abstract
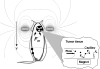
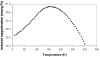
Magnetic targeting is a promising strategy for achieving localized drug delivery. Application of this strategy to treat brain tumors, however, is complicated by their deep intracranial location, since magnetic field density cannot be focused at a distance from an externally applied magnet. This study intended to examine whether, with magnetic targeting, pathological alteration in brain tumor flow dynamics could be of value in discriminating the diseased site from healthy brain. To address this question, the capture of magnetic nanoparticles was first assessed in vitro using a simple flow system under theoretically estimated glioma and normal brain flow conditions. Secondly, accumulation of nanoparticles via magnetic targeting was evaluated in vivo using 9L-glioma bearing rats. In vitro results that predicted a 7.6-fold increase in nanoparticle capture at glioma- versus contralateral brain-relevant flow rates were relatively consistent with the 9.6-fold glioma selectivity of nanoparticle accumulation over the contralateral brain observed in vivo. Based on these finding, the in vitro ratio of nanoparticle capture can be viewed as a plausible indicator of in vivo glioma selectivity. Overall, it can be concluded that the decreased blood flow rate in glioma, reflecting tumor vascular abnormalities, is an important contributor to glioma-selective nanoparticle accumulation with magnetic targeting.
Figures




Similar articles
-
Brain tumor targeting of magnetic nanoparticles for potential drug delivery: effect of administration route and magnetic field topography.J Control Release. 2011 Nov 7;155(3):393-9. doi: 10.1016/j.jconrel.2011.06.033. Epub 2011 Jul 7. J Control Release. 2011. PMID: 21763736 Free PMC article.
-
Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors.Biomaterials. 2008 Feb;29(4):487-96. doi: 10.1016/j.biomaterials.2007.08.050. Epub 2007 Oct 26. Biomaterials. 2008. PMID: 17964647 Free PMC article.
-
Gum arabic-coated magnetic nanoparticles for potential application in simultaneous magnetic targeting and tumor imaging.AAPS J. 2009 Dec;11(4):693-9. doi: 10.1208/s12248-009-9151-y. Epub 2009 Oct 20. AAPS J. 2009. PMID: 19842043 Free PMC article.
-
Blood flow and blood-to-tissue transport in 9L gliosarcomas: the role of the brain tumor model in drug delivery research.J Neurooncol. 1991 Dec;11(3):185-97. doi: 10.1007/BF00165526. J Neurooncol. 1991. PMID: 1823340 Review.
-
Chlorotoxin-conjugated nanoparticles for targeted imaging and therapy of glioma.Curr Top Med Chem. 2015;15(13):1196-208. doi: 10.2174/1568026615666150330110822. Curr Top Med Chem. 2015. PMID: 25858130 Review.
Cited by
-
Magnetic nanoparticles as targeted delivery systems in oncology.Radiol Oncol. 2011 Mar;45(1):1-16. doi: 10.2478/v10019-011-0001-z. Epub 2011 Jan 19. Radiol Oncol. 2011. PMID: 22933928 Free PMC article.
-
Magnetic resonance monitoring of focused ultrasound/magnetic nanoparticle targeting delivery of therapeutic agents to the brain.Proc Natl Acad Sci U S A. 2010 Aug 24;107(34):15205-10. doi: 10.1073/pnas.1003388107. Epub 2010 Aug 9. Proc Natl Acad Sci U S A. 2010. PMID: 20696897 Free PMC article.
-
Dendrimer- and copolymer-based nanoparticles for magnetic resonance cancer theranostics.Theranostics. 2018 Nov 29;8(22):6322-6349. doi: 10.7150/thno.27828. eCollection 2018. Theranostics. 2018. PMID: 30613300 Free PMC article. Review.
-
Magnetic Nanoparticles in the Central Nervous System: Targeting Principles, Applications and Safety Issues.Molecules. 2017 Dec 21;23(1):9. doi: 10.3390/molecules23010009. Molecules. 2017. PMID: 29267188 Free PMC article. Review.
-
Improving Tumor Retention of Effector Cells in Adoptive Cell Transfer Therapies by Magnetic Targeting.Pharmaceutics. 2020 Aug 27;12(9):812. doi: 10.3390/pharmaceutics12090812. Pharmaceutics. 2020. PMID: 32867162 Free PMC article. Review.
References
-
- Rautio J, Chikhale PJ. Drug delivery systems for brain tumor therapy. Curr Pharm Des. 2004;10(12):1341–1353. - PubMed
-
- Hafeli UO, Sweeney SM, Beresford BA, Humm JL, Macklis RM. Effective targeting of magnetic radioactive 90Y-microspheres to tumor cells by an externally applied magnetic field. Preliminary in vitro and in vivo results. Nucl Med Biol. 1995;22(2):147–55. - PubMed
-
- Alexiou C, Arnold W, Klein RJ, Parak FG, Hulin P, Bergemann C, Erhardt W, Wagenpfeil S, Lubbe AS. Locoregional cancer treatment with magnetic drug targeting. Cancer Res. 2000;60(23):6641–8. - PubMed
-
- Alexiou C, Jurgons R, Schmid RJ, Bergemann C, Henke J, Erhardt W, Huenges E, Parak F. Magnetic drug targeting--biodistribution of the magnetic carrier and the chemotherapeutic agent mitoxantrone after locoregional cancer treatment. J Drug Target. 2003;11(3):139–49. - PubMed
-
- Hatch G, Stelter R. Magnetic design considerations for devices and particles used for biological high-gradient magnetic separation (HGMS) systems. J Magn Magn Mater. 2001;225:272–76.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

