Nanolayer biomaterial coatings of silk fibroin for controlled release
- PMID: 17628161
- PMCID: PMC2695962
- DOI: 10.1016/j.jconrel.2007.06.006
Nanolayer biomaterial coatings of silk fibroin for controlled release
Abstract
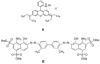
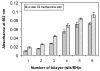
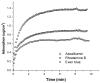
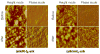
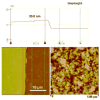
An all-aqueous, stepwise deposition process with silk fibroin protein for the assembly of nanoscale layered controlled release coatings was exploited. Model compounds, Rhodamine B, Even Blue and Azoalbumin, representing small molecule drugs and therapeutically relevant proteins were incorporated in the nanocoating process and their loading and release behavior was quantified. In addition, the structure and morphology of the coatings were characterized. Release studies in vitro showed that control of beta-sheet crystal content and the multilayer structure of the silk coatings correlated with the release properties of the incorporated compounds. In particular, higher crystallinity and a thicker silk capping layer suppressed the initial burst of release and prolonged the duration of release. These novel coatings and deposition approach provide a unique option to regulate structure and morphology, and thus release kinetics. The results also suggest these systems as a promising framework for surface engineering of biomaterials and medical devices to regulate the release of drugs, when considered with the all-aqueous process involved, the conformal nature of the coatings, the robust material properties of silk fibroin, and the degradability and biocompatibility of this family of protein.
Figures



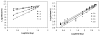
References
-
- Fredin NJ, Zhang J, Lynn DM. Surface analysis of erodible multilayered polyelectrolyte films: nanometer-scale structure and erosion profiles. Langmuir. 2005;21(13):5803–5811. - PubMed
-
- Forzani ES, Perez MA, Lopez Teijelo M, Calvo EJ. Redox driven swelling of layer-by-layer enzyme-polyelectrolyte multilayers. Langmuir. 2002;18(25):9867–9873.
-
- Thierry B, Winnik FM, Merhi Y, Silver J, Tabrizian M. Bioactive coatings of endovascular stents based on polyelectrolyte multilayers. Biomacromolecules. 2003;4:1564–1571. - PubMed
-
- Hammond P. Form and function in multilayer assembly: new applications at the nanoscale. Adv Mater. 2004;16:1271–1293.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

