Delta and Egfr expression are regulated by Importin-7/Moleskin in Drosophila wing development
- PMID: 17628519
- PMCID: PMC1994573
- DOI: 10.1016/j.ydbio.2007.06.011
Delta and Egfr expression are regulated by Importin-7/Moleskin in Drosophila wing development
Abstract
Drosophila DIM-7 (encoded by the moleskin gene, msk) is the orthologue of vertebrate Importin-7. Both Importin-7 and Msk/DIM-7 function as nuclear import cofactors, and have been implicated in the control of multiple signal transduction pathways, including the direct nuclear import of the activated (phosphorylated) form of MAP kinase. We performed two genetic deficiency screens to identify deficiencies that similarly modified Msk overexpression phenotypes in both eyes and wings. We identified 11 total deficiencies, one of which removes the Delta locus. In this report, we show that Delta loss-of-function alleles dominantly suppress Msk gain-of-function phenotypes in the developing wing. We find that Msk overexpression increases both Delta protein expression and Delta transcription, though Msk expression alone is not sufficient to activate Delta protein function. We also find that Msk overexpression increases Egfr protein levels, and that msk gene function is required for proper Egfr expression in both developing wings and eyes. These results indicate a novel function for Msk in Egfr expression. We discuss the implications of these data with respect to the integration of Egfr and Delta/Notch signaling, specifically through the control of MAP kinase subcellular localization.
Figures


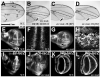
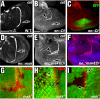
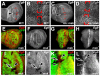
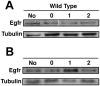
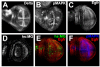
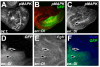
Similar articles
-
Moleskin is essential for the formation of the myotendinous junction in Drosophila.Dev Biol. 2011 Nov 15;359(2):176-89. doi: 10.1016/j.ydbio.2011.08.028. Epub 2011 Sep 9. Dev Biol. 2011. PMID: 21925492 Free PMC article.
-
A genetic screen in Drosophila for genes interacting with senseless during neuronal development identifies the importin moleskin.Genetics. 2007 Jan;175(1):125-41. doi: 10.1534/genetics.106.065680. Epub 2006 Nov 16. Genetics. 2007. PMID: 17110483 Free PMC article.
-
Mutual repression by bantam miRNA and Capicua links the EGFR/MAPK and Hippo pathways in growth control.Curr Biol. 2012 Apr 24;22(8):651-7. doi: 10.1016/j.cub.2012.02.050. Epub 2012 Mar 22. Curr Biol. 2012. PMID: 22445297
-
Selector and signalling molecules cooperate in organ patterning.Nat Cell Biol. 2002 Mar;4(3):E48-51. doi: 10.1038/ncb0302-e48. Nat Cell Biol. 2002. PMID: 11875444 Review.
-
Intercellular signaling and the polarization of body axes during Drosophila oogenesis.Genes Dev. 1996 Jul 15;10(14):1711-23. doi: 10.1101/gad.10.14.1711. Genes Dev. 1996. PMID: 8698232 Review. No abstract available.
Cited by
-
Vibrio parahaemolyticus VopA Is a Potent Inhibitor of Cell Migration and Apoptosis in the Intestinal Epithelium of Drosophila melanogaster.Infect Immun. 2019 Feb 21;87(3):e00669-18. doi: 10.1128/IAI.00669-18. Print 2019 Mar. Infect Immun. 2019. PMID: 30617204 Free PMC article.
-
Association of the Protein-Quality-Control Protein Ubiquilin-1 With Alzheimer's Disease Both in vitro and in vivo.Front Neurosci. 2022 Mar 17;16:821059. doi: 10.3389/fnins.2022.821059. eCollection 2022. Front Neurosci. 2022. PMID: 35401099 Free PMC article.
-
The Drosophila histone variant H2Av facilitates Notch signaling activity in a two-tier regulatory fashion.Cell Commun Signal. 2025 Jul 1;23(1):322. doi: 10.1186/s12964-025-02333-6. Cell Commun Signal. 2025. PMID: 40598498 Free PMC article.
References
-
- Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995;268:225–232. - PubMed
-
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
-
- Baker NE. Transcription of the segment-polarity gene wingless in the imaginal discs of Drosophila, and the phenotype of a pupal-lethal wg mutation. Development. 1988;102:489–497. - PubMed
-
- Baron M. An overview of the Notch signalling pathway. Semin Cell Dev Biol. 2003;14:113–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

