Mago Nashi and Tsunagi/Y14, respectively, regulate Drosophila germline stem cell differentiation and oocyte specification
- PMID: 17628520
- PMCID: PMC3010412
- DOI: 10.1016/j.ydbio.2007.06.007
Mago Nashi and Tsunagi/Y14, respectively, regulate Drosophila germline stem cell differentiation and oocyte specification
Abstract
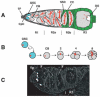
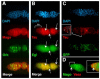
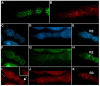
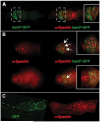
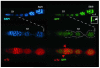
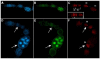
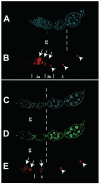
A protein complex consisting of Mago Nashi and Tsunagi/Y14 is required to establish the major body axes and for the localization of primordial germ cell determinants during Drosophila melanogaster oogenesis. The Mago Nashi:Tsunagi/Y14 heterodimer also serves as the core of the exon junction complex (EJC), a multiprotein complex assembled on spliced mRNAs. In previous studies, reduced function alleles of mago nashi and tsunagi/Y14 were used to characterize the roles of the genes in oogenesis. Here, we investigated mago nashi and tsunagi/Y14 using null alleles and clonal analysis. Germline clones lacking mago nashi function divide but fail to differentiate. The mago nashi null germline stem cells produce clones over a period of at least 11 days, suggesting that mago nashi is not necessary for stem cell self-renewal. However, germline stem cells lacking tsunagi/Y14 function are indistinguishable from wild type. Additionally, in tsunagi/Y14 null germline cysts, centrosomes and oocyte-specific components fail to concentrate within a single cell and oocyte fate is not restricted to a single cell. Together, our results suggest not only that mago nashi is required for germline stem cell differentiation but that surprisingly mago nashi functions independently of tsunagi/Y14 in this process. On the other hand, Tsunagi/Y14 is essential for restricting oocyte fate to a single cell and may function with mago nashi in this process.
Figures

References
-
- Andersen CB, et al. Structure of the exon junction core complex with a trapped DEAD-box ATPase bound to RNA. Science. 2006;313:1968–1972. - PubMed
-
- Ballut L, et al. The exon junction core complex is locked onto RNA by inhibition of eIF4AIII ATPase activity. Nat. Struct. Mol. Biol. 2005;12:861–869. - PubMed
-
- Bolivar J, et al. Centrosome migration into the Drosophila oocyte is independent of BicD and egl, and of the organisation of the microtubule cytoskeleton. Development. 2001;128:889–1897. - PubMed
-
- Bono F, et al. The crystal structure of the exon junction complex reveals how it maintains a stable grip on mRNA. Cell. 2006;126:713–725. - PubMed
-
- Boswell RE, et al. Mutations in a newly identified Drosophila melanogaster gene, mago nashi, disrupt germ cell formation and result in the formation of mirror-image symmetrical double abdomen embryos. Development. 1991;113:373–384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

