Flow cytometric detection of degranulation reveals phenotypic heterogeneity of degranulating CMV-specific CD8+ T lymphocytes in rhesus macaques
- PMID: 17628586
- PMCID: PMC2039909
- DOI: 10.1016/j.jim.2007.05.011
Flow cytometric detection of degranulation reveals phenotypic heterogeneity of degranulating CMV-specific CD8+ T lymphocytes in rhesus macaques
Abstract
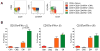
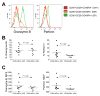
Flow-cytometric conditions for detection of lysosomal-associated membrane proteins (LAMPs) on the surface of recently degranulated cells were optimized for rhesus macaques and used to investigate the functional properties of rhesus cytomegalovirus (rhCMV)-specific CD8+ T lymphocytes with regards to cytotoxicity and interferon (IFN)-gamma secretion in six asymptomatic CMV-seropositive rhesus macaques. Unlike humans, the rhesus macaque LAMP-1 protein CD107a underwent little or no endocytosis over a six to 18 h stimulation period. Following in vitro stimulation, rhCMV-specific CD8+ T lymphocytes were heterogeneous with regards to the composition of cells positive for CD107a and/or IFN-gamma, time to reach peak degranulation, and kinetics of IFN-gamma secretion relative to degranulation. Responder CD8+ T lymphocytes that underwent degranulation without IFN-gamma production (CD107a+IFN-gamma-) were predominantly composed of terminally differentiated effectors (CD28-CD45RA+). Moreover, they had significantly lower frequencies of effector memory (CD28-CD45RA-) cells compared to the IFN-gamma-secreting cells that did or did not undergo degranulation (CD107a+IFN-gamma+ or CD107a-IFN-gamma+). The perforin content of effector CD8+ T lymphocytes was significantly greater than that of effector memory CD8+ T lymphocytes in rhesus macaques, suggesting that they were more cytolytic. Our findings suggest that the composition of rhCMV-specific CD8+ T lymphocytes with regards to CD107a+IFN-gamma- responders may be an important determinant of their ability to control CMV replication.
Figures

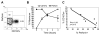



References
-
- Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. - PubMed
-
- . The Institute of Laboratory Animal Resources. National Research Council: Guide for the Care and Use of Laboratory Animals. 1996. pp. 86–123.
-
- Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, Little S, Havlir DV, Richman DD, Gruener N, Pape G, Waters A, Easterbrook P, Salio M, Cerundolo V, McMichael AJ, Rowland-Jones SL. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–85. - PubMed
-
- Baars PA, Ribeiro Do Couto LM, Leusen JH, Hooibrink B, Kuijpers TW, Lens SM, van Lier RA. Cytolytic mechanisms and expression of activation-regulating receptors on effector-type CD8+CD45RA+CD27- human T cells. J Immunol. 2000;165:1910–7. - PubMed
-
- Bachmann MF, Barner M, Viola A, Kopf M. Distinct kinetics of cytokine production and cytolysis in effector and memory T cells after viral infection. Eur J Immunol. 1999;29:291–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

