Addressing challenges in adolescent smoking cessation: design and baseline characteristics of the HS Group-Randomized trial
- PMID: 17628650
- PMCID: PMC2040060
- DOI: 10.1016/j.ypmed.2007.05.018
Addressing challenges in adolescent smoking cessation: design and baseline characteristics of the HS Group-Randomized trial
Abstract
Objective: Well-documented challenges have hampered both intervention development and research in teen smoking cessation. Addressing these challenges, the Hutchinson Study of High School Smoking (HS Study), the largest group-randomized trial in adolescent smoking cessation to date, incorporates several design innovations to investigate the effect of a counselor-initiated, individually tailored telephone counseling smoking cessation intervention for older adolescents. This paper presents and discusses these innovative design features, and baseline findings on the resulting study population.
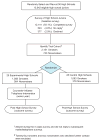
Method: The trial used a population-based survey to proactively identify and recruit all high school juniors who had smoked in the past month - potentially expanding intervention reach to all smokers, even those who smoked less than daily and those not motivated to quit. For ethical and intervention reasons, some nonsmokers were enrolled in the intervention, also. Other important design features included the random allocation of schools into experimental conditions (intervention vs. no-intervention control) and a multi-wave design.
Results and conclusion: The design innovations address problems and challenges identified in adolescent smoking cessation literature. The heterogeneous baseline characteristics of the study population, well-balanced between the two arms, have three significant implications: They (1) demonstrate the effectiveness of the trial's design features, (2) highlight several intervention-related issues, and (3) provide assurance that the trial's evaluation of intervention effectiveness will be unbiased.
References
-
- Allen KF, Moss AJ, Giovino GA, Shopland DR, Pierce JP. Teenage tobacco use: data estimates from the Teenage Attitudes and Practices Survey, United States, 1989. Advance Data. 1993;224:1–20. - PubMed
-
- Altman DG. Comparability of randomized groups. Statistician. 1985;34:125–136.
-
- Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet. 2000;335:1064–1069. - PubMed
-
- Bachman JG, Wadsworth KN, O’Malley PM, Johnston LD, Schulenberg JE. Smoking, Drinking, and Drug Use in Young Adulthood: The Impacts of New Freedoms and New Responsibilities. Mahwah, NJ: Lawrence Erlbaum Associates; 1997.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous