Key connexin 43 phosphorylation events regulate the gap junction life cycle
- PMID: 17629739
- PMCID: PMC2596931
- DOI: 10.1007/s00232-007-9035-y
Key connexin 43 phosphorylation events regulate the gap junction life cycle
Abstract
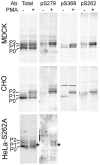
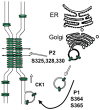
Connexin 43 (Cx43), the most widely expressed and abundant vertebrate gap junction protein, is phosphorylated at multiple different serine residues during its life cycle. Cx43 is phosphorylated soon after synthesis and phosphorylation changes as it traffics through the endoplasmic reticulum and Golgi to the plasma membrane, ultimately forming a gap junction structure. The electrophoretic mobility of Cx43 changes as the protein proceeds through its life cycle, with prominent bands often labeled P0, P1 and P2. Many reports have indicated changes in "phosphorylation" based on these mobility shifts and others that occur in response to growth factors or other biological effectors. Here, we indicate how phosphospecific and epitope-specific antibodies can be utilized to show when and where certain phosphorylation events occur during the Cx43 life cycle. These reagents show that phosphorylation at S364 and/or S365 is involved in forming the P1 isoform, an event that apparently regulates trafficking to or within the plasma membrane. Phosphorylation at S325, S328 and/or S330 is necessary to form a P2 isoform; and this phosphorylation event is present only in gap junctions. Treatment with protein kinase C activators led to phosphorylation at S368, S279/S282 and S262 with a shift in mobility in CHO, but not MDCK, cells. The shift was dependent on mitogen-activated protein kinase activity but not phosphorylation at S279/S282. However, phosphorylation at S262 could explain the shift. By defining these phosphorylation events, we have begun to sort out the critical signaling pathways that regulate gap junction function.
Figures


Similar articles
-
Phosphorylation of serine 262 in the gap junction protein connexin-43 regulates DNA synthesis in cell-cell contact forming cardiomyocytes.J Cell Sci. 2004 Jan 26;117(Pt 3):507-14. doi: 10.1242/jcs.00889. J Cell Sci. 2004. PMID: 14702389
-
Regulation of epidermal growth factor-induced connexin 43 gap junction communication by big mitogen-activated protein kinase1/ERK5 but not ERK1/2 kinase activation.J Biol Chem. 2003 May 16;278(20):18682-8. doi: 10.1074/jbc.M213283200. Epub 2003 Mar 12. J Biol Chem. 2003. PMID: 12637502
-
Connexin 43 in LA-25 cells with active v-src is phosphorylated on Y247, Y265, S262, S279/282, and S368 via multiple signaling pathways.Cell Commun Adhes. 2008 May;15(1):75-84. doi: 10.1080/15419060802014016. Cell Commun Adhes. 2008. PMID: 18649180 Free PMC article.
-
Ubiquitination of gap junction proteins.J Membr Biol. 2007 Jun;217(1-3):43-51. doi: 10.1007/s00232-007-9050-z. Epub 2007 Jul 28. J Membr Biol. 2007. PMID: 17657522 Review.
-
Connexin phosphorylation as a regulatory event linked to gap junction channel assembly.Biochim Biophys Acta. 2005 Jun 10;1711(2):154-63. doi: 10.1016/j.bbamem.2004.09.013. Epub 2004 Oct 12. Biochim Biophys Acta. 2005. PMID: 15955300 Review.
Cited by
-
Posttranslational modifications in connexins and pannexins.J Membr Biol. 2012 Jun;245(5-6):319-32. doi: 10.1007/s00232-012-9453-3. Epub 2012 Jun 28. J Membr Biol. 2012. PMID: 22739962 Free PMC article. Review.
-
Acetylation mediates Cx43 reduction caused by electrical stimulation.J Mol Cell Cardiol. 2015 Oct;87:54-64. doi: 10.1016/j.yjmcc.2015.08.001. Epub 2015 Aug 8. J Mol Cell Cardiol. 2015. PMID: 26264759 Free PMC article.
-
Local effects and mechanisms of antiarrhythmic peptide AAP10 in acute regional myocardial ischemia: electrophysiological and molecular findings.Naunyn Schmiedebergs Arch Pharmacol. 2008 Nov;378(5):459-70. doi: 10.1007/s00210-008-0317-4. Epub 2008 Jun 24. Naunyn Schmiedebergs Arch Pharmacol. 2008. PMID: 18574580
-
The connexin 46 mutant (V44M) impairs gap junction function causing congenital cataract.J Genet. 2017 Dec;96(6):969-976. doi: 10.1007/s12041-017-0861-0. J Genet. 2017. PMID: 29321356
-
Serine 319 phosphorylation is necessary and sufficient to induce a Cx37 conformation that leads to arrested cell cycling.J Cell Sci. 2020 Jun 18;133(12):jcs240721. doi: 10.1242/jcs.240721. J Cell Sci. 2020. PMID: 32350069 Free PMC article.
References
-
- Beardslee MA, Lerner DL, Tadros PN, Laing JG, Beyer EC, Yamada KA, Kleber AG, Schuessler RB, Saffitz JE. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res. 2000;87:656–662. - PubMed
-
- Cooper CD, Lampe PD. Casein kinase 1 regulates connexin43 gap junction assembly. J Biol Chem. 2002;277:44962–44968. - PubMed
-
- Doble BW, Dang X, Ping P, Fandrich RR, Nickel BE, Jin Y, Cattini PA, Kardami E. Phosphorylation of serine 262 in the gap junction protein connexin-43 regulates DNA synthesis in cell-cell contact forming cardiomyocytes. J Cell Sci. 2004;117:507–14. - PubMed
-
- Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

