Expression of the Ku70 subunit (XRCC6) and protection from low dose ionizing radiation during zebrafish embryogenesis
- PMID: 17630212
- PMCID: PMC2075087
- DOI: 10.1016/j.neulet.2007.05.045
Expression of the Ku70 subunit (XRCC6) and protection from low dose ionizing radiation during zebrafish embryogenesis
Abstract
The Ku70 protein, a product of the XRCC6 gene, is a component of the nonhomologous end-joining (NHEJ) pathway of DNA repair, which protects cells from the effects of radiation-induced DNA damage. Although the spatial expression of Ku70 during vertebrate embryogenesis has not been described, DNA repair proteins are generally considered to be "housekeeping" genes, which are required for radioprotection in all cells. Here, we report the cloning and characterization of the zebrafish Ku70 ortholog. In situ hybridization and RT-PCR analyses demonstrate that Ku70 mRNA is maternally provided and expressed uniformly among embryonic blastomeres. Later during embryogenesis, zygotically transcribed Ku70 mRNA specifically accumulates in neural tissue, including the retina and proliferative regions of the developing brain. In the absence of genotoxic stress, morpholino-mediated knockdown of Ku70 expression does not affect zebrafish embryogenesis. However, exposure of Ku70 morpholino-injected embryos to low doses of ionizing radiation leads to marked cell death throughout the developing brain, spinal cord, and tail. These results suggest that Ku70 protein plays a crucial role in protecting the developing nervous system from radiation-induced DNA damage during embryogenesis.
Figures
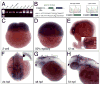

References
-
- Ward JF. Radiation mutagenesis: the initial DNA lesions responsible. Radiat Res. 1995;142(3):362–368. - PubMed
-
- Hefferin ML, Tomkinson AE. Mechanism of DNA double-strand break repair by non-homologous end joining. DNA Repair (Amst) 2005;4(6):639–48. - PubMed
-
- Wyman C, Ristic D, Kanaar R. Homologous recombination-mediated double-strand break repair. DNA Repair (Amst) 2004;3(89):827–33. - PubMed
-
- Getts RC, Stamato TD. Absence of a Ku-like DNA end binding activity in the xrs double-strand DNA repair-deficient mutant. J Biol Chem. 1994;269(23):15981–15984. - PubMed
-
- Smider V, Rathmell WK, Lieber MR, Chu G. Restoration of X-ray resistance and V(D)J recombination in mutant cells by Ku cDNA. Science. 1994;266(5183):288–291. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

