Diesel-enriched particulate matter functionally activates human dendritic cells
- PMID: 17630318
- PMCID: PMC2219549
- DOI: 10.1165/rcmb.2007-0199OC
Diesel-enriched particulate matter functionally activates human dendritic cells
Abstract
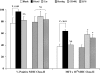
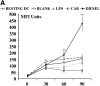
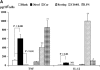
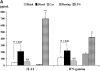
Epidemiologic studies have associated exposure to airborne particulate matter (PM) with exacerbations of asthma. It is unknown how different sources of PM affect innate immunity. We sought to determine how car- and diesel exhaust-derived PM affects dendritic cell (DC) activation. DC development was modeled using CD34+ hematopoietic progenitors. Airborne PM was collected from exhaust plenums of Fort McHenry Tunnel providing car-enriched particles (CEP) and diesel-enriched particles (DEP). DC were stimulated for 48 hours with CEP, DEP, CD40-ligand, or lipopolysaccharide. DC activation was assessed by flow cytometry, enzyme-linked immunosorbent assay, and standard culture techniques. DEP increased uptake of fluorescein isothiocyanate-dextran (a model antigen) by DC. Diesel particles enhanced cell-surface expression of co-stimulatory molecules (e.g., CD40 [P < 0.01] and MHC class II [P < 0.01]). By contrast, CEP poorly affected antigen uptake and expression of cell surface molecules, and did not greatly affect cytokine secretion by DC. However, DEP increased production of TNF, IL-6, and IFN-gamma (P < 0.01), IL-12 (P < 0.05), and vascular endothelial growth factor (P < 0.001). In co-stimulation assays of PM-exposed DC and alloreactive CD4+ T cells, both CEP and DEP directed a Th2-like pattern of cytokine production (e.g., enhanced IL-13 and IL-18 and suppressed IFN-gamma production). CD4+ T cells were not functionally activated on exposure to either DEP or CEP. Car- and diesel-enriched particles exert a differential effect on DC activation. Our data support the hypothesis that DEP (and to a lesser extent CEP) regulate important functional aspects of human DC, supporting an adjuvant role for this material.
Figures




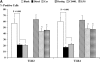
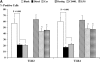








Similar articles
-
Ambient particulate matter directs nonclassic dendritic cell activation and a mixed TH1/TH2-like cytokine response by naive CD4+ T cells.J Allergy Clin Immunol. 2007 Feb;119(2):488-97. doi: 10.1016/j.jaci.2006.10.022. Epub 2006 Dec 21. J Allergy Clin Immunol. 2007. PMID: 17187851
-
Activation of human monocyte-derived dendritic cells in vitro by the biological response modifier arabinoxylan rice bran (MGN-3/Biobran).Int J Immunopathol Pharmacol. 2011 Oct-Dec;24(4):941-8. doi: 10.1177/039463201102400412. Int J Immunopathol Pharmacol. 2011. PMID: 22230400
-
Effects of diesel exhaust particles on antigen-presenting cells and antigen-specific Th immunity in mice.Exp Biol Med (Maywood). 2009 Feb;234(2):200-9. doi: 10.3181/0809-RM-285. Epub 2008 Dec 8. Exp Biol Med (Maywood). 2009. PMID: 19064938
-
Causation by Diesel Exhaust Particles of Endothelial Dysfunctions in Cytotoxicity, Pro-inflammation, Permeability, and Apoptosis Induced by ROS Generation.Cardiovasc Toxicol. 2017 Oct;17(4):384-392. doi: 10.1007/s12012-016-9364-0. Cardiovasc Toxicol. 2017. PMID: 26965709 Review.
-
Biology of diesel exhaust effects on allergic pulmonary inflammation.Yakugaku Zasshi. 2011 Mar;131(3):367-71. doi: 10.1248/yakushi.131.367. Yakugaku Zasshi. 2011. PMID: 21372531 Review.
Cited by
-
Diesel exhaust particle exposure reduces expression of the epithelial tight junction protein Tricellulin.Part Fibre Toxicol. 2020 Oct 15;17(1):52. doi: 10.1186/s12989-020-00383-x. Part Fibre Toxicol. 2020. PMID: 33059747 Free PMC article.
-
Particulate matter-induced airway hyperresponsiveness is lymphocyte dependent.Environ Health Perspect. 2010 May;118(5):640-6. doi: 10.1289/ehp.0901461. Epub 2010 Jan 8. Environ Health Perspect. 2010. PMID: 20061214 Free PMC article.
-
Regulation of COX-2 expression and IL-6 release by particulate matter in airway epithelial cells.Am J Respir Cell Mol Biol. 2009 Jan;40(1):19-30. doi: 10.1165/rcmb.2008-0105OC. Epub 2008 Jul 10. Am J Respir Cell Mol Biol. 2009. PMID: 18617679 Free PMC article.
-
Engineered silica nanoparticles act as adjuvants to enhance allergic airway disease in mice.Part Fibre Toxicol. 2013 Jul 1;10:26. doi: 10.1186/1743-8977-10-26. Part Fibre Toxicol. 2013. PMID: 23815813 Free PMC article.
-
Diesel exhaust exposure and nasal response to attenuated influenza in normal and allergic volunteers.Am J Respir Crit Care Med. 2012 Jan 15;185(2):179-85. doi: 10.1164/rccm.201103-0465OC. Epub 2011 Oct 27. Am J Respir Crit Care Med. 2012. PMID: 22071326 Free PMC article. Clinical Trial.
References
-
- Dominici F, McDermott A, Daniels M, Zeger SL, Samet JM. Revised analyses of the National Morbidity, Mortality, and Air Pollution Study: mortality among residents of 90 cities. J Toxicol Environ Health A 2005;68:1071–1092. - PubMed
-
- Ryan PH, LeMasters G, Biagini J, Bernstein D, Grinshpun SA, Shukla R, Wilson K, Villareal M, Burkle J, Lockey J. Is it traffic type, volume, or distance? Wheezing in infants living near truck and bus traffic. J Allergy Clin Immunol 2005;116:279–284. - PubMed
-
- Nel AE, Diaz-Sanchez D, Ng D, Hiura T, Saxon A. Enhancement of allergic inflammation by the interaction of between diesel exhaust particles and the immune system. J Allergy Clin Immunol 1998;102:539–554. - PubMed
-
- Riedl M, Diaz-Sanchez D. Biology of diesel exhaust effects on respiratory function. J Allergy Clin Immunol 2005;115:221–228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

