EhLimA, a novel LIM protein, localizes to the plasma membrane in Entamoeba histolytica
- PMID: 17630327
- PMCID: PMC2043370
- DOI: 10.1128/EC.00177-07
EhLimA, a novel LIM protein, localizes to the plasma membrane in Entamoeba histolytica
Abstract
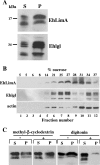
The parasitic protozoan Entamoeba histolytica relies on a very dynamic cytoskeleton in order to invade and survive in host tissues. Identification of cytoskeletal elements is key to understanding these processes. Here we present the characterization of EhLimA, the first LIM protein of E. histolytica. EhLimA consists of a single LIM domain at its N terminus and exhibits the highest degree of homology with DdLimE from Dictyostelium discoideum. Immunofluorescence localization of EhLimA using anti-EhLimA antibodies revealed that EhLimA is highly concentrated at the plasma membrane of cells. Silencing or overexpression of the EhLimA gene did not have a significant effect on the growth or morphology of the parasite. EhLimA associates with the cytoskeleton as demonstrated by the enrichment of the protein in cytoskeleton fractions as well as in pull-down assays that revealed that cytoskeleton association involves interaction with actin. EhLimA binding to actin was shown to be dependent on the N-terminal LIM domain of EhLimA, as removal of even half of the LIM domain resulted in almost complete inhibition of the binding to actin. We also found that a portion of EhLimA floats to the lower-density regions of a sucrose gradient together with portions of the Gal-lectin light subunit and actin. Treatment of cells with the cholesterol-sequestering agent digitonin resulted in increased solubility of EhLimA. These results indicate that in addition to cytoskeletal association, EhLimA may also associate with lipid rafts in the parasite plasma membrane and suggest that EhLimA may be part of the molecular system connecting the actin cytoskeleton to membrane rafts.
Figures







Similar articles
-
Molecular and functional characterization of an Entamoeba histolytica protein (EhMLCI) with features of a myosin essential light chain.Mol Biochem Parasitol. 2012 Jan;181(1):17-28. doi: 10.1016/j.molbiopara.2011.09.007. Epub 2011 Sep 24. Mol Biochem Parasitol. 2012. PMID: 21963788
-
EhNCABP166: a nucleocytoplasmic actin-binding protein from Entamoeba histolytica.Mol Biochem Parasitol. 2010 Jul;172(1):19-30. doi: 10.1016/j.molbiopara.2010.03.010. Epub 2010 Mar 23. Mol Biochem Parasitol. 2010. PMID: 20338197
-
Rab7 and actin cytoskeleton participate during mobilization of β1EHFNR in fibronectin-stimulated Entamoeba histolyticatrophozoites.Microsc Res Tech. 2012 Mar;75(3):285-93. doi: 10.1002/jemt.21056. Epub 2011 Aug 5. Microsc Res Tech. 2012. PMID: 21823201
-
Cytoskeleton-membrane interactions in membrane raft structure.Cell Mol Life Sci. 2009 Jul;66(14):2319-28. doi: 10.1007/s00018-009-0022-6. Epub 2009 Apr 16. Cell Mol Life Sci. 2009. PMID: 19370312 Free PMC article. Review.
-
Nuclear transport in Entamoeba histolytica: knowledge gap and therapeutic potential.Parasitology. 2018 Sep;145(11):1378-1387. doi: 10.1017/S0031182018000252. Epub 2018 Mar 22. Parasitology. 2018. PMID: 29565001 Review.
Cited by
-
Host-Parasite interactions in Entamoeba histolytica and Entamoeba dispar: what have we learned from their genomes?Parasite Immunol. 2012 Feb-Mar;34(2-3):90-9. doi: 10.1111/j.1365-3024.2011.01325.x. Parasite Immunol. 2012. PMID: 21810102 Free PMC article. Review.
-
A landscape of gene regulation in the parasitic amoebozoa Entamoeba spp.PLoS One. 2022 Aug 1;17(8):e0271640. doi: 10.1371/journal.pone.0271640. eCollection 2022. PLoS One. 2022. PMID: 35913975 Free PMC article.
-
The cell surface proteome of Entamoeba histolytica.Mol Cell Proteomics. 2014 Jan;13(1):132-44. doi: 10.1074/mcp.M113.031393. Epub 2013 Oct 17. Mol Cell Proteomics. 2014. PMID: 24136294 Free PMC article.
-
New insights into Entamoeba histolytica pathogenesis.Curr Opin Infect Dis. 2008 Oct;21(5):489-94. doi: 10.1097/QCO.0b013e32830ce75f. Curr Opin Infect Dis. 2008. PMID: 18725798 Free PMC article. Review.
-
Knockdown of Five Genes Encoding Uncharacterized Proteins Inhibits Entamoeba histolytica Phagocytosis of Dead Host Cells.Infect Immun. 2016 Mar 24;84(4):1045-1053. doi: 10.1128/IAI.01325-15. Print 2016 Apr. Infect Immun. 2016. PMID: 26810036 Free PMC article.
References
-
- Ankri, S., F. Padilla-Vaca, T. Stolarsky, L. Koole, U. Katz, and D. Mirelman. 1999. Antisense inhibition of expression of the light subunit (35 kDa) of the Gal/GalNac lectin complex inhibits Entamoeba histolytica virulence. Mol. Microbiol. 33:327-337. - PubMed
-
- Bach, I. 2000. The LIM domain: regulation by association. Mech. Dev. 91:5-17. - PubMed
-
- Baltz, R., C. Domon, D. T. Pillay, and A. Steinmetz. 1992. Characterization of a pollen-specific cDNA from sunflower encoding a zinc finger protein. Plant J. 2:713-721. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

