Direct evidence for specific interactions of the fibrinogen alphaC-domains with the central E region and with each other
- PMID: 17630702
- PMCID: PMC2678904
- DOI: 10.1021/bi700944j
Direct evidence for specific interactions of the fibrinogen alphaC-domains with the central E region and with each other
Abstract
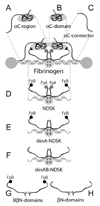
The carboxyl-terminal regions of the fibrinogen Aalpha chains (alphaC regions) form compact alphaC-domains tethered to the bulk of the molecule with flexible alphaC-connectors. It was hypothesized that in fibrinogen two alphaC-domains interact intramolecularly with each other and with the central E region preferentially through its N-termini of Bbeta chains and that removal of fibrinopeptides A and B upon fibrin assembly results in dissociation of the alphaC regions and their switch to intermolecular interactions. To test this hypothesis, we studied the interactions of the recombinant alphaC region (Aalpha221-610 fragment) and its subfragments, alphaC-connector (Aalpha221-391) and alphaC-domain (Aalpha392-610), between each other and with the recombinant (Bbeta1-66)2 and (beta15-66)2 fragments and NDSK corresponding to the fibrin(ogen) central E region, using laser tweezers-based force spectroscopy. The alphaC-domain, but not the alphaC-connector, bound to NDSK, which contains fibrinopeptides A and B, and less frequently to desA-NDSK and (Bbeta1-66)2 containing only fibrinopeptides B; it was poorly reactive with desAB-NDSK and (beta15-66)2 both lacking fibrinopeptide B. The interactions of the alphaC-domains with each other and with the alphaC-connector were also observed, although they were weaker and heterogeneous in strength. These results provide the first direct evidence for the interaction between the alphaC-domains and the central E region through fibrinopeptide B, in agreement with the hypothesis given above, and indicate that fibrinopeptide A is also involved. They also confirm the hypothesized homomeric interactions between the alphaC-domains and display their interaction with the alphaC-connectors, which may contribute to covalent cross-linking of alpha polymers in fibrin.
Figures



References
-
- Weisel JW. Fibrinogen and fibrin. Adv. Protein Chem. 2005;70:247–299. - PubMed
-
- Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3:1894–1904. - PubMed
-
- Blomback B, Blomback M, Henschen A, Hessel B, Iwanaga S, Woods KR. N-terminal disulphide knot of human fibrinogen. Nature. 1968;218:130–134. - PubMed
-
- Yang Z, Kollman JM, Pandi L, Doolittle RF. Crystal structure of native chicken fibrinogen at 2.7 A resolution. Biochemistry. 2001;40:12515–12523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

