Sulfate as a synergistic anion facilitating iron binding by the bacterial transferrin FbpA: the origins and effects of anion promiscuity
- PMID: 17630737
- PMCID: PMC3674819
- DOI: 10.1021/ja0709268
Sulfate as a synergistic anion facilitating iron binding by the bacterial transferrin FbpA: the origins and effects of anion promiscuity
Abstract
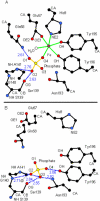
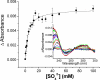
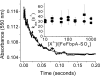
The ferric binding protein, FbpA, has been demonstrated to facilitate the transport of naked Fe3+ across the periplasmic space of several Gram-negative bacteria. The sequestration of iron by FbpA is facilitated by the presence of a synergistic anion, such as phosphate or sulfate. Here we report the sequestration of Fe3+ by FbpA in the presence of sulfate, at an assumed periplasmic pH of 6.5 to form FeFbpA-SO4 with K'(eff) = 1.7 x 10(16) M(-1) (at 20 degrees C, 50 mM MES, 200 mM KCl). The iron affinity of the FeFbpA-SO4 protein assembly is 2 orders of magnitude lower than when bound with phosphate and is the lowest of any of the FeFbpA-X assemblies yet reported. Iron reduction at the cytosolic membrane receptor may be an essential aspect of the periplasmic iron-transport process, and with an E(1/2) of -158 mV (NHE), FeFbpA-SO4 is the most easily reduced of all FeFbpA-X assemblies yet studied. The variation of FeFbpA-X assembly stability (K'(eff)) and ease of reduction (E(1/2)) with differing synergistic anions X(n-) are correlated over a range of 14 kJ, suggesting that the variations in redox potentials are due to stabilization of Fe3+ in FeFbpA-X by X(n-). Anion promiscuity of FbpA in the diverse composition of the periplasmic space is illustrated by the ex vivo exchange kinetics of FeFbpA-SO4 with phosphate and arsenate, where first-order kinetics with respect to FeFbpA-SO4 (k = 30 s(-1)) are observed at pH 6.5, independent of entering anion concentration and identity. Anion lability and influence on the iron affinity and reduction potential for FeFbpA-X support the hypothesis that synergistic anion exchange may be an important regulator in iron delivery to the cytosol. This structural and thermodynamic analysis of anion binding in FeFbpA-X provides additional insight into anion promiscuity and importance.
Figures






References
-
- Crichton RR. Inorganic Biochemistry of Iron Metabolism: From Molecular Mechanism to Clinical Consequences. 2nd ed. Wiley; New York: 2001.
-
- Crosa JH, Mey AR, Payne SM. Iron Transport in Bacteria. ASM Press; Washington D.C.: 2004.
-
- Mietzner TA, Tencza SB, Adhikari P, Vaughan KG, Nowalk AJ. In: Current Topics Microbiology and Immunology. Vogt PK, Mahan MJ, editors. Vol. 225. Springer; Berlin: 1998. pp. 113–35. - PubMed
-
- Sritharan M. World J. Microbiol. Biotechnol. 2000;16:769–80.
-
- Clarke TE, Tari LW, Vogel HJ. Curr. Top. Med. Chem. 2001;1:7–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

