Multi-minicore Disease
- PMID: 17631035
- PMCID: PMC1947955
- DOI: 10.1186/1750-1172-2-31
Multi-minicore Disease
Abstract
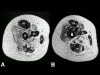
Multi-minicore Disease (MmD) is a recessively inherited neuromuscular disorder characterized by multiple cores on muscle biopsy and clinical features of a congenital myopathy. Prevalence is unknown. Marked clinical variability corresponds to genetic heterogeneity: the most instantly recognizable classic phenotype characterized by spinal rigidity, early scoliosis and respiratory impairment is due to recessive mutations in the selenoprotein N (SEPN1) gene, whereas recessive mutations in the skeletal muscle ryanodine receptor (RYR1) gene have been associated with a wider range of clinical features comprising external ophthalmoplegia, distal weakness and wasting or predominant hip girdle involvement resembling central core disease (CCD). In the latter forms, there may also be a histopathologic continuum with CCD due to dominant RYR1 mutations, reflecting the common genetic background. Pathogenetic mechanisms of RYR1-related MmD are currently not well understood, but likely to involve altered excitability and/or changes in calcium homeoestasis; calcium-binding motifs within the selenoprotein N protein also suggest a possible role in calcium handling. The diagnosis of MmD is based on the presence of suggestive clinical features and multiple cores on muscle biopsy; muscle MRI may aid genetic testing as patterns of selective muscle involvement are distinct depending on the genetic background. Mutational analysis of the RYR1 or the SEPN1 gene may provide genetic confirmation of the diagnosis. Management is mainly supportive and has to address the risk of marked respiratory impairment in SEPN1-related MmD and the possibility of malignant hyperthermia susceptibility in RYR1-related forms. In the majority of patients, weakness is static or only slowly progressive, with the degree of respiratory impairment being the most important prognostic factor.
Figures

References
-
- Engel AG, Gomez MR, Groover RV. Multicore disease. Mayo Clin Proc. 1971;10:666–681. - PubMed
-
- Wallgren-Pettersson C. Congenital nemaline myopathy: a longitudinal study. Vol. 30. Academic dissertation, University of Helsinki, Commentationes Physico-Mathematicae 111/Dissertationes; 1990. p. 102.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

