Effector role of neonatal hepatic CD8+ lymphocytes in epithelial injury and autoimmunity in experimental biliary atresia
- PMID: 17631148
- PMCID: PMC2013308
- DOI: 10.1053/j.gastro.2007.04.031
Effector role of neonatal hepatic CD8+ lymphocytes in epithelial injury and autoimmunity in experimental biliary atresia
Abstract
Background & aims: Lymphocytes populate the livers of infants with biliary atresia, but it is unknown whether neonatal lymphocytes regulate pathogenesis of disease. Here, we investigate this question by examining the role of T lymphocytes in the destruction of extrahepatic bile ducts of neonatal mice using an experimental model of biliary atresia.
Methods: Inoculation of neonatal mice with rhesus rotavirus followed by multistaining flow cytometry to quantify expression of interferon-gamma by hepatic lymphocytes, and real-time polymerase chain reaction for mRNA expression of pro-inflammatory cytokines. This was followed by determining the consequences of antibody-mediated depletion of lymphocyte subtypes on the development of biliary obstruction, and coculture and cell transfer experiments to investigate the effector role of lymphocyte subtypes on neonatal biliary disease.
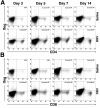
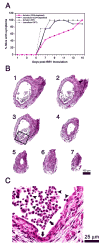
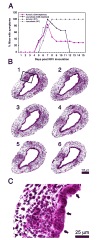
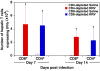
Results: Rotavirus infection results in overexpression of interferon-gamma by neonatal hepatic T cells. Among these cells, depletion of CD4(+) cells did not change the course of inflammatory injury and obstruction of neonatal bile ducts. In contrast, loss of CD8(+) cells remarkably suppressed duct injury, prevented luminal obstruction, and restored bile flow. Coculture experiments showed that rotavirus-primed, but not naïve, CD8(+) cells were cytotoxic to cholangiocytes. In adoptive transfer experiments, we found that primed CD8(+) cells preferentially homed to extrahepatic bile ducts of neonatal mice and invaded their epithelial lining.
Conclusions: Primed neonatal CD8(+) cells can activate a pro-inflammatory program, target diseased and healthy duct epithelium, and drive the phenotypic expression of biliary atresia, thus constituting a potential therapeutic target to halt disease progression.
Figures


References
-
- Garcia AM, Fadel SA, Cao S, Sarzotti M. T cell immunity in neonates. Immunol Res. 2000;22:177–90. - PubMed
-
- Adkins B. Development of neonatal Th1/Th2 function. Int Rev Immunol. 2000;19:157–71. - PubMed
-
- Bot A, Bot S, Bona C. Enhanced protection against influenza virus of mice immunized as newborns with a mixture of plasmids expressing hemagglutinin and nucleoprotein. Vaccine. 1998;16:1675–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

