Glucan, water dikinase activity stimulates breakdown of starch granules by plastidial beta-amylases
- PMID: 17631522
- PMCID: PMC1976587
- DOI: 10.1104/pp.107.104224
Glucan, water dikinase activity stimulates breakdown of starch granules by plastidial beta-amylases
Abstract
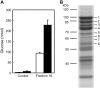
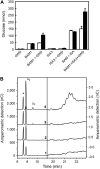
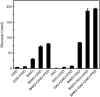
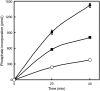
Glucan phosphorylating enzymes are required for normal mobilization of starch in leaves of Arabidopsis (Arabidopsis thaliana) and potato (Solanum tuberosum), but mechanisms underlying this dependency are unknown. Using two different activity assays, we aimed to identify starch degrading enzymes from Arabidopsis, whose activity is affected by glucan phosphorylation. Breakdown of granular starch by a protein fraction purified from leaf extracts increased approximately 2-fold if the granules were simultaneously phosphorylated by recombinant potato glucan, water dikinase (GWD). Using matrix-assisted laser-desorption ionization mass spectrometry several putative starch-related enzymes were identified in this fraction, among them beta-AMYLASE1 (BAM1; At3g23920) and ISOAMYLASE3 (ISA3; At4g09020). Experiments using purified recombinant enzymes showed that BAM1 activity with granules similarly increased under conditions of simultaneous starch phosphorylation. Purified recombinant potato ISA3 (StISA3) did not attack the granular starch significantly with or without glucan phosphorylation. However, starch breakdown by a mixture of BAM1 and StISA3 was 2 times higher than that by BAM1 alone and was further enhanced in the presence of GWD and ATP. Similar to BAM1, maltose release from granular starch by purified recombinant BAM3 (At4g17090), another plastid-localized beta-amylase isoform, increased 2- to 3-fold if the granules were simultaneously phosphorylated by GWD. BAM activity in turn strongly stimulated the GWD-catalyzed phosphorylation. The interdependence between the activities of GWD and BAMs offers an explanation for the severe starch excess phenotype of GWD-deficient mutants.
Figures


Similar articles
-
The plastidial glucan, water dikinase (GWD) catalyses multiple phosphotransfer reactions.FEBS J. 2012 Jun;279(11):1953-66. doi: 10.1111/j.1742-4658.2012.08576.x. Epub 2012 Apr 25. FEBS J. 2012. PMID: 22429449
-
Phosphorylation of transitory starch by α-glucan, water dikinase during starch turnover affects the surface properties and morphology of starch granules.New Phytol. 2014 Jul;203(2):495-507. doi: 10.1111/nph.12801. Epub 2014 Apr 3. New Phytol. 2014. PMID: 24697163
-
Identification of a novel enzyme required for starch metabolism in Arabidopsis leaves. The phosphoglucan, water dikinase.Plant Physiol. 2005 Jan;137(1):242-52. doi: 10.1104/pp.104.055954. Epub 2004 Dec 23. Plant Physiol. 2005. PMID: 15618411 Free PMC article.
-
Leaf starch degradation comes out of the shadows.Trends Plant Sci. 2005 Mar;10(3):130-7. doi: 10.1016/j.tplants.2005.01.001. Trends Plant Sci. 2005. PMID: 15749471 Review.
-
Starch phosphorylation: insights and perspectives.Cell Mol Life Sci. 2016 Jul;73(14):2753-64. doi: 10.1007/s00018-016-2248-4. Epub 2016 May 4. Cell Mol Life Sci. 2016. PMID: 27147464 Free PMC article. Review.
Cited by
-
Influence of parboiling conditions on rice grain quality characters and insect infestation with rice weevil (Sitophilus oryzae. L) of some rice cultivars.BMC Plant Biol. 2024 Oct 17;24(1):978. doi: 10.1186/s12870-024-05651-y. BMC Plant Biol. 2024. PMID: 39420297 Free PMC article.
-
Physiological and Transcriptional Responses of Apocynum venetum to Salt Stress at the Seed Germination Stage.Int J Mol Sci. 2023 Feb 11;24(4):3623. doi: 10.3390/ijms24043623. Int J Mol Sci. 2023. PMID: 36835035 Free PMC article.
-
Uncovering the genetic basis for quality traits in the Mediterranean old wheat germplasm and phenotypic and genomic prediction assessment by cross-validation test.Front Plant Sci. 2023 Jan 27;14:1127357. doi: 10.3389/fpls.2023.1127357. eCollection 2023. Front Plant Sci. 2023. PMID: 36778676 Free PMC article.
-
TgLaforin, a glucan phosphatase, reveals the dynamic role of storage polysaccharides in Toxoplasma gondii tachyzoites and bradyzoites.bioRxiv [Preprint]. 2024 Nov 8:2023.09.29.560185. doi: 10.1101/2023.09.29.560185. bioRxiv. 2024. PMID: 37808860 Free PMC article. Preprint.
-
Assessing the impacts of genetic defects on starch metabolism in Arabidopsis plants using the carbon homeostasis model.J R Soc Interface. 2023 Nov;20(208):20230426. doi: 10.1098/rsif.2023.0426. Epub 2023 Nov 29. J R Soc Interface. 2023. PMID: 38016639 Free PMC article.
References
-
- Alonso-Casajús N, Dauvillée D, Viale AM, Munoz J, Baroja-Fernández E, Morán-Zorzano MT, Eydallin G, Ball S, Pozueta-Romero J (2006) Glycogen phosphorylase, the product of the glgP gene, catalyzes glycogen breakdown by removing glucose units from the nonreducing ends in Escherichia coli. J Bacteriol 188 5266–5272 - PMC - PubMed
-
- Ball SG, Morell MK (2003) From bacterial glycogen to starch: understanding the biogenisis of the starch granule. Annu Rev Plant Biol 54 207–233 - PubMed
-
- Baunsgaard L, Lütken H, Mikkelsen R, Glaring MA, Pham TT, Blennow A (2005) A novel isoform of glucan, water dikinase phosphorylates pre-phosphorylated α-glucans and is involved in starch degradation in Arabidopsis. Plant J 41 595–695 - PubMed
-
- Blennow A, Engelsen SB, Nielsen TH, Baunsgaard L, Mikkelsen R (2002) Starch phosphorylation: a new front line in starch research. Trends Plant Sci 7 445–450 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

