Phosphate homeostasis and root development in Arabidopsis are synchronized by the zinc finger transcription factor ZAT6
- PMID: 17631527
- PMCID: PMC1976576
- DOI: 10.1104/pp.107.101691
Phosphate homeostasis and root development in Arabidopsis are synchronized by the zinc finger transcription factor ZAT6
Abstract
Phosphorus availability is limited in many natural ecosystems. Plants adapt to phosphate (Pi) deficiency by complex molecular processes. There is growing evidence suggesting that transcription factors are key components in the regulation of these processes. In this study, we characterized the function of ZAT6 (zinc finger of Arabidopsis 6), a cysteine-2/histidine-2 zinc finger transcription factor that is responsive to Pi stress. ZAT6 is induced during Pi starvation and localizes to the nucleus. While the RNAi suppression of ZAT6 appeared to be lethal, its overexpression affects root development and retards seedling growth as a result of decreased Pi acquisition. The ZAT6 overexpression also resulted in altered root architecture of older plants, with consequent changes in Pi acquisition. These results indicate that ZAT6 regulates root development independent of the Pi status of the plant, thereby influencing Pi acquisition and homeostasis. In addition, the expression of several Pi starvation-responsive genes was decreased in ZAT6 overexpressing plants, thereby confirming the role of ZAT6 in regulating Pi homeostasis. This study thus indicates that ZAT6 is a repressor of primary root growth and regulates Pi homeostasis through the control of root architecture. To our knowledge, ZAT6 is the first cysteine-2/histidine-2 zinc finger transcription factor reported to regulate root development and nutrient stress responses.
Figures


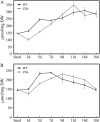






References
-
- Abel S, Ticconi AC, Delatorre CA (2002) Phosphate sensing in higher plants. Physiol Plant 115 1–8 - PubMed
-
- Abramoff MD, Magelhaes PJ, Ram SJ (2004) Image processing with ImageJ. Biophotonics International 11 36–42
-
- Ascencio J (1994) Acid phosphatase as a diagnostic tool. Commun Soil Sci Plant Anal 25 1553–1564
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

