Nanopipette delivery of individual molecules to cellular compartments for single-molecule fluorescence tracking
- PMID: 17631532
- PMCID: PMC2025666
- DOI: 10.1529/biophysj.107.104737
Nanopipette delivery of individual molecules to cellular compartments for single-molecule fluorescence tracking
Abstract
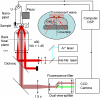
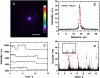
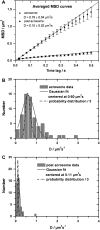
We have developed a new method, using a nanopipette, for controlled voltage-driven delivery of individual fluorescently labeled probe molecules to the plasma membrane which we used for single-molecule fluorescence tracking (SMT). The advantages of the method are 1), application of the probe to predefined regions on the membrane; 2), release of only one or a few molecules onto the cell surface; 3), when combined with total internal reflection fluorescence microscopy, very low background due to unbound molecules; and 4), the ability to first optimize the experiment and then repeat it on the same cell. We validated the method by performing an SMT study of the diffusion of individual membrane glycoproteins labeled with Atto 647-wheat germ agglutin in different surface domains of boar spermatozoa. We found little deviation from Brownian diffusion with a mean diffusion coefficient of 0.79 +/- 0.04 microm(2)/s in the acrosomal region and 0.10 +/- 0.02 microm(2)/s in the postacrosomal region; this difference probably reflects different membrane structures. We also showed that we can analyze diffusional properties of different subregions of the cell membrane and probe for the presence of diffusion barriers. It should be straightforward to extend this new method to other probes and cells, and it can be used as a new tool to investigate the cell membrane.
Figures

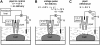

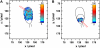
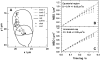

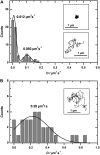
References
-
- Kusumi, A., C. Nakada, K. Ritchie, K. Murase, K. Suzuki, H. Murakoshi, R. S. Kasai, J. Kondo, and T. Fujiwara. 2005. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu. Rev. Biophys. Biomol. Struct. 34:351–378. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

