The role of mitochondria in protection of the heart by preconditioning
- PMID: 17631856
- PMCID: PMC2212780
- DOI: 10.1016/j.bbabio.2007.05.008
The role of mitochondria in protection of the heart by preconditioning
Abstract
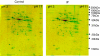
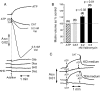
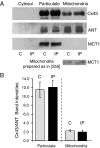
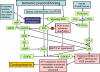
A prolonged period of ischaemia followed by reperfusion irreversibly damages the heart. Such reperfusion injury (RI) involves opening of the mitochondrial permeability transition pore (MPTP) under the conditions of calcium overload and oxidative stress that accompany reperfusion. Protection from MPTP opening and hence RI can be mediated by ischaemic preconditioning (IP) where the prolonged ischaemic period is preceded by one or more brief (2-5 min) cycles of ischaemia and reperfusion. Following a brief overview of the molecular characterisation and regulation of the MPTP, the proposed mechanisms by which IP reduces pore opening are reviewed including the potential roles for reactive oxygen species (ROS), protein kinase cascades, and mitochondrial potassium channels. It is proposed that IP-mediated inhibition of MPTP opening at reperfusion does not involve direct phosphorylation of mitochondrial proteins, but rather reflects diminished oxidative stress during prolonged ischaemia and reperfusion. This causes less oxidation of critical thiol groups on the MPTP that are known to sensitise pore opening to calcium. The mechanisms by which ROS levels are decreased in the IP hearts during prolonged ischaemia and reperfusion are not known, but appear to require activation of protein kinase Cepsilon, either by receptor-mediated events or through transient increases in ROS during the IP protocol. Other signalling pathways may show cross-talk with this primary mechanism, but we suggest that a role for mitochondrial potassium channels is unlikely. The evidence for their activity in isolated mitochondria and cardiac myocytes is reviewed and the lack of specificity of the pharmacological agents used to implicate them in IP is noted. Some K(+) channel openers uncouple mitochondria and others inhibit respiratory chain complexes, and their ability to produce ROS and precondition hearts is mimicked by bona fide uncouplers and respiratory chain inhibitors. IP may also provide continuing protection during reperfusion by preventing a cascade of MPTP-induced ROS production followed by further MPTP opening. This phase of protection may involve survival kinase pathways such as Akt and glycogen synthase kinase 3 (GSK3) either increasing ROS removal or reducing mitochondrial ROS production.
Figures

References
-
- Halestrap A.P., Kerr P.M., Javadov S., Woodfield K.Y. Elucidating the molecular mechanism of the permeability transition pore and its role in reperfusion injury of the heart. Biochim. Biophys. Acta. 1998;1366:79–94. - PubMed
-
- Suleiman M.S., Halestrap A.P., Griffiths E.J. Mitochondria: a target for myocardial protection. Pharmacol. Ther. 2001;89:29–46. - PubMed
-
- Halestrap A.P., Clarke S.J., Javadov S.A. Mitochondrial permeability transition pore opening during myocardial reperfusion—A target for cardioprotection. Cardiovasc. Res. 2004;61:372–385. - PubMed
-
- Downey J.M., Cohen M.V. Reducing infarct size in the setting of acute myocardial infarction. Prog. Cardiovasc. Dis. 2006;48:363–371. - PubMed
-
- Fliss H., Gattinger D. Apoptosis in ischemic and reperfused rat myocardium. Circ. Res. 1996;79:949–956. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

