The interferon-induced expression of APOBEC3G in human blood-brain barrier exerts a potent intrinsic immunity to block HIV-1 entry to central nervous system
- PMID: 17631933
- PMCID: PMC2737467
- DOI: 10.1016/j.virol.2007.06.010
The interferon-induced expression of APOBEC3G in human blood-brain barrier exerts a potent intrinsic immunity to block HIV-1 entry to central nervous system
Abstract
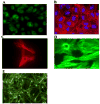
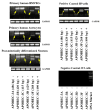
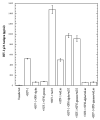
In the human genome, the APOBEC3 gene has expanded into a tandem array of genes termed APOBEC3A-H. Several members of this family have potent anti-HIV-1 activity. Here we demonstrate that APOBEC-3B/3C/3F and -3G are expressed in all major cellular components of the CNS. Moreover, we show that both interferon-alpha (IFN-alpha) and IFN-gamma significantly enhance the expression of APOBEC-3G/3F and drastically inhibit HIV-1 replication in primary human brain microvascular endothelial cells (BMVECs), the major component of blood-brain barrier (BBB). As the viral inhibition can be neutralized by APOBEC3G-specific siRNA, APOBEC3G plays a key role to mediate the anti-HIV-1 activity of IFN-alpha and/or IFN-gamma. Our findings suggest that, in addition to the restriction at viral entry level, the restriction from APOBEC3 family could account for the low-level replication of HIV-1 in BMVECs. The manipulation of IFN-APOBEC3 signaling pathway could be a potent therapeutic strategy to prevent HIV invasion to central nervous system (CNS).
Figures

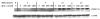
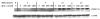
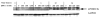
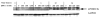


References
-
- Acheampong E, Parveen Z, Mengistu A, Ngoubilly N, Wigdahl B, Lossinsky AS, Pomerantz RJ, Mukhtar M. Cholesterol-depleting statin drugs protect postmitotically differentiated human neurons against ethanol- and human immunodeficiency virus type 1-induced oxidative stress in vitro. J Virol. 2007;81(3):1492–501. - PMC - PubMed
-
- Bagasra O, Lavi E, Bobroski L, Khalili K, Pestaner JP, Tawadros R, Pomerantz RJ. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. Aids. 1996;10(6):573–85. - PubMed
-
- Bell JE, Busuttil A, Ironside JW, Rebus S, Donaldson YK, Simmonds P, Peutherer JF. Human immunodeficiency virus and the brain: investigation of virus load and neuropathologic changes in pre-AIDS subjects. J Infect Dis. 1993;168(4):818–24. - PubMed
-
- Berglund O, Engman K, Ehrnst A, Andersson J, Lidman K, Akerlund B, Sonnerborg A, Strannegard O. Combined treatment of symptomatic human immunodeficiency virus type 1 infection with native interferon-alpha and zidovudine. J Infect Dis. 1991;163(4):710–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

