Stability and nuclear dynamics of the bicoid morphogen gradient
- PMID: 17632061
- PMCID: PMC2253672
- DOI: 10.1016/j.cell.2007.05.026
Stability and nuclear dynamics of the bicoid morphogen gradient
Abstract
Patterning in multicellular organisms results from spatial gradients in morphogen concentration, but the dynamics of these gradients remain largely unexplored. We characterize, through in vivo optical imaging, the development and stability of the Bicoid morphogen gradient in Drosophila embryos that express a Bicoid-eGFP fusion protein. The gradient is established rapidly (approximately 1 hr after fertilization), with nuclear Bicoid concentration rising and falling during mitosis. Interphase levels result from a rapid equilibrium between Bicoid uptake and removal. Initial interphase concentration in nuclei in successive cycles is constant (+/-10%), demonstrating a form of gradient stability, but it subsequently decays by approximately 30%. Both direct photobleaching measurements and indirect estimates of Bicoid-eGFP diffusion constants (D < or = 1 microm(2)/s) provide a consistent picture of Bicoid transport on short ( approximately min) time scales but challenge traditional models of long-range gradient formation. A new model is presented emphasizing the possible role of nuclear dynamics in shaping and scaling the gradient.
Figures
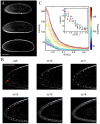

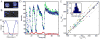
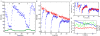


Comment in
-
Bicoid by the numbers: quantifying a morphogen gradient.Cell. 2007 Jul 13;130(1):14-6. doi: 10.1016/j.cell.2007.06.036. Cell. 2007. PMID: 17632050
-
Re-examining the stability of the Bicoid morphogen gradient.Cell. 2008 Jan 11;132(1):15-7; author reply 17-8. doi: 10.1016/j.cell.2007.12.022. Cell. 2008. PMID: 18191212 No abstract available.
References
-
- Ariizumi T, Asashima M. Control of the embryonic body plan by activin during amphibian development. Zoolog Sci. 1995;12:509–521. - PubMed
-
- Barolo S, Carver LA, Posakony JW. GFP and beta-galactosidase transformation vectors for promoter/enhancer analysis in Drosophila. Biotechniques. 2000;29:726–732. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

