Protein kinase C protects preconditioned rabbit hearts by increasing sensitivity of adenosine A2b-dependent signaling during early reperfusion
- PMID: 17632123
- PMCID: PMC2729547
- DOI: 10.1016/j.yjmcc.2007.05.016
Protein kinase C protects preconditioned rabbit hearts by increasing sensitivity of adenosine A2b-dependent signaling during early reperfusion
Abstract
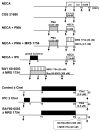
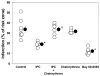
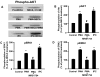
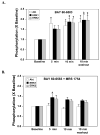
Although protein kinase C (PKC) plays a key role in ischemic preconditioning (IPC), the actual mechanism of that protection is unknown. We recently found that protection from IPC requires activation of adenosine receptors during early reperfusion. We, therefore, hypothesized that PKC might act to increase the heart's sensitivity to adenosine. IPC limited infarct size in isolated rabbit hearts subjected to 30-min regional ischemia/2-h reperfusion and IPC's protection was blocked by the PKC inhibitor chelerythrine given during early reperfusion revealing involvement of PKC at reperfusion. Similarly chelerythrine infused in the early reperfusion period blocked the increased phosphorylation of the protective kinases Akt and ERK1/2 observed after IPC. Infusing phorbol 12-myristate 13-acetate (PMA), a PKC activator, during early reperfusion mimicked IPC's protection. As expected, the protection triggered by PMA at reperfusion was blocked by chelerythrine, but surprisingly it was also blocked by MRS1754, an adenosine A(2b) receptor-selective antagonist, suggesting that PKC was somehow facilitating signaling from the A(2b) receptors. NECA [5'-(N-ethylcarboxamido) adenosine], a potent but not selective A(2b) receptor agonist, increased phosphorylation of Akt and ERK1/2 in a dose-dependent manner. Pretreating hearts with PMA or brief preconditioning ischemia had no effect on phosphorylation of Akt or ERK1/2 per se but markedly lowered the threshold for NECA to induce their phosphorylation. BAY 60-6583, a highly selective A(2b) agonist, also caused phosphorylation of ERK1/2 and Akt. MRS1754 prevented phosphorylation induced by BAY 60-6583. BAY 60-6583 limited infarct size when given to ischemic hearts at reperfusion. These results suggest that activation of cardiac A(2b) receptors at reperfusion is protective, but because of the very low affinity of the receptors endogenous cardiac adenosine is unable to trigger their signaling. We propose that the key protective event in IPC occurs when PKC increases the heart's sensitivity to adenosine so that endogenous adenosine can activate A(2b)-dependent signaling.
Figures




References
-
- Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol. 2005;288:H971–H976. - PubMed
-
- Philipp S, Yang X-M, Cui L, Davis AM, Downey JM, Cohen MV. Postconditioning protects rabbit hearts through a protein kinase C-adenosine A2b receptor cascade. Cardiovasc Res. 2006;70:308–314. - PubMed
-
- Yang X-M, Proctor JB, Cui L, Krieg T, Downey JM, Cohen MV. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J Am Coll Cardiol. 2004;44:1103–1110. - PubMed
-
- Solenkova NV, Solodushko V, Cohen MV, Downey JM. Endogenous adenosine protects preconditioned heart during early minutes of reperfusion by activating Akt. Am J Physiol. 2006;290:H441–H449. - PubMed
-
- Kin H, Zatta AJ, Lofye MT, Amerson BS, Halkos ME, Kerendi F, et al. Postconditioning reduces infarct size via adenosine receptor activation by endogenous adenosine. Cardiovasc Res. 2005;67:124–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

