Viral RNA pseudoknots: versatile motifs in gene expression and replication
- PMID: 17632571
- PMCID: PMC7096944
- DOI: 10.1038/nrmicro1704
Viral RNA pseudoknots: versatile motifs in gene expression and replication
Abstract
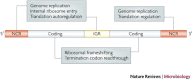
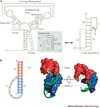
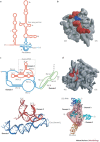
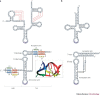
RNA pseudoknots are structural elements found in almost all classes of RNA. First recognized in the genomes of plant viruses, they are now established as a widespread motif with diverse functions in various biological processes. This Review focuses on viral pseudoknots and their role in virus gene expression and genome replication. Although emphasis is placed on those well defined pseudoknots that are involved in unusual mechanisms of viral translational initiation and elongation, the broader roles of pseudoknots are also discussed, including comparisons with relevant cellular counterparts. The relationship between RNA pseudoknot structure and function is also addressed.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
A genome-wide analysis of RNA pseudoknots that stimulate efficient -1 ribosomal frameshifting or readthrough in animal viruses.Biomed Res Int. 2013;2013:984028. doi: 10.1155/2013/984028. Epub 2013 Nov 4. Biomed Res Int. 2013. PMID: 24298557 Free PMC article.
-
Comparative studies of frameshifting and nonframeshifting RNA pseudoknots: a mutational and NMR investigation of pseudoknots derived from the bacteriophage T2 gene 32 mRNA and the retroviral gag-pro frameshift site.RNA. 2002 Aug;8(8):981-96. doi: 10.1017/s1355838202024044. RNA. 2002. PMID: 12212853 Free PMC article.
-
Diverse roles and interactions of RNA structures during the replication of positive-stranded RNA viruses of humans and animals.J Gen Virol. 2015 Jul;96(Pt 7):1497-503. doi: 10.1099/vir.0.000066. Epub 2015 Jan 27. J Gen Virol. 2015. PMID: 25626680 Review.
-
Solution structure of the pseudoknot of SRV-1 RNA, involved in ribosomal frameshifting.J Mol Biol. 2001 Jul 27;310(5):1109-23. doi: 10.1006/jmbi.2001.4823. J Mol Biol. 2001. PMID: 11501999 Free PMC article.
-
RNA pseudoknots and the regulation of protein synthesis.Biochem Soc Trans. 2008 Aug;36(Pt 4):684-9. doi: 10.1042/BST0360684. Biochem Soc Trans. 2008. PMID: 18631140 Review.
Cited by
-
Structures and Functions of the 3' Untranslated Regions of Positive-Sense Single-Stranded RNA Viruses Infecting Humans and Animals.Front Cell Infect Microbiol. 2020 Aug 27;10:453. doi: 10.3389/fcimb.2020.00453. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32974223 Free PMC article. Review.
-
The HCV IRES pseudoknot positions the initiation codon on the 40S ribosomal subunit.RNA. 2010 Aug;16(8):1559-69. doi: 10.1261/rna.2197210. Epub 2010 Jun 28. RNA. 2010. PMID: 20584896 Free PMC article.
-
RAG: an update to the RNA-As-Graphs resource.BMC Bioinformatics. 2011 May 31;12:219. doi: 10.1186/1471-2105-12-219. BMC Bioinformatics. 2011. PMID: 21627789 Free PMC article.
-
RNA elements in open reading frames of the bluetongue virus genome are essential for virus replication.PLoS One. 2014 Mar 21;9(3):e92377. doi: 10.1371/journal.pone.0092377. eCollection 2014. PLoS One. 2014. PMID: 24658296 Free PMC article.
-
Structural basis of differential ligand recognition by two classes of bis-(3'-5')-cyclic dimeric guanosine monophosphate-binding riboswitches.Proc Natl Acad Sci U S A. 2011 May 10;108(19):7757-62. doi: 10.1073/pnas.1018857108. Epub 2011 Apr 25. Proc Natl Acad Sci U S A. 2011. PMID: 21518891 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

