Pbx homeodomain proteins pattern both the zebrafish retina and tectum
- PMID: 17634100
- PMCID: PMC1934912
- DOI: 10.1186/1471-213X-7-85
Pbx homeodomain proteins pattern both the zebrafish retina and tectum
Abstract
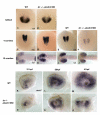
Background: Pbx genes encode TALE class homeodomain transcription factors that pattern the developing neural tube, pancreas, and blood. Within the hindbrain, Pbx cooperates with Hox proteins to regulate rhombomere segment identity. Pbx cooperates with Eng to regulate midbrain-hindbrain boundary maintenance, and with MyoD to control fast muscle cell differentiation. Although previous results have demonstrated that Pbx is required for proper eye size, functions in regulating retinal cell identity and patterning have not yet been examined.
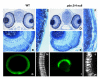
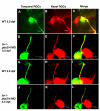
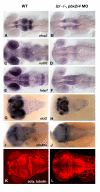
Results: Analysis of retinal ganglion cell axon pathfinding and outgrowth in pbx2/4 null embryos demonstrated a key role for pbx genes in regulating neural cell behavior. To identify Pbx-dependent genes involved in regulating retino-tectal pathfinding, we conducted a microarray screen for Pbx-dependent transcripts in zebrafish, and detected genes that are specifically expressed in the eye and tectum. A subset of Pbx-dependent retinal transcripts delineate specific domains in the dorso-temporal lobe of the developing retina. Furthermore, we determined that some Pbx-dependent transcripts also require Meis1 and Gdf6a function. Since gdf6a expression is also dependent on Pbx, we propose a model in which Pbx proteins regulate expression of the growth factor gdf6a, which in turn regulates patterning of the dorso-temporal lobe of the retina. This, in concert with aberrant tectal patterning in pbx2/4 null embryos, may lead to the observed defects in RGC outgrowth.
Conclusion: These data define a novel role for Pbx in patterning the vertebrate retina and tectum in a manner required for proper retinal ganglion cell axon outgrowth.
Figures


Similar articles
-
Meis1 specifies positional information in the retina and tectum to organize the zebrafish visual system.Neural Dev. 2010 Sep 1;5:22. doi: 10.1186/1749-8104-5-22. Neural Dev. 2010. PMID: 20809932 Free PMC article.
-
Pbx homeodomain proteins direct Myod activity to promote fast-muscle differentiation.Development. 2007 Sep;134(18):3371-82. doi: 10.1242/dev.003905. Epub 2007 Aug 15. Development. 2007. PMID: 17699609
-
astray, a zebrafish roundabout homolog required for retinal axon guidance.Science. 2001 Apr 20;292(5516):507-10. doi: 10.1126/science.1059496. Science. 2001. PMID: 11313496
-
[Morphogenesis of the optic tectum and retinotectal map formation].Tanpakushitsu Kakusan Koso. 2000 Feb;45(3 Suppl):316-22. Tanpakushitsu Kakusan Koso. 2000. PMID: 10707636 Review. Japanese. No abstract available.
-
TALE transcription factors during early development of the vertebrate brain and eye.Dev Dyn. 2014 Jan;243(1):99-116. doi: 10.1002/dvdy.24030. Epub 2013 Sep 18. Dev Dyn. 2014. PMID: 23939996 Review.
Cited by
-
Meis1 regulates Foxn4 expression during retinal progenitor cell differentiation.Biol Open. 2013 Sep 6;2(11):1125-36. doi: 10.1242/bio.20132279. eCollection 2013. Biol Open. 2013. PMID: 24244849 Free PMC article.
-
Coordinating progenitor cell cycle exit and differentiation in the developing vertebrate retina.Neurogenesis (Austin). 2016 Apr 11;3(1):e1161697. doi: 10.1080/23262133.2016.1161697. eCollection 2016. Neurogenesis (Austin). 2016. PMID: 27604453 Free PMC article.
-
A Comprehensive Drosophila melanogaster Transcription Factor Interactome.Cell Rep. 2019 Apr 16;27(3):955-970.e7. doi: 10.1016/j.celrep.2019.03.071. Cell Rep. 2019. PMID: 30995488 Free PMC article.
-
Conservation of gene linkage in dispersed vertebrate NK homeobox clusters.Dev Genes Evol. 2009 Oct;219(9-10):481-96. doi: 10.1007/s00427-009-0311-y. Dev Genes Evol. 2009. PMID: 20112453
-
500,000 fish phenotypes: The new informatics landscape for evolutionary and developmental biology of the vertebrate skeleton.J Appl Ichthyol. 2012 Jun 1;28(3):300-305. doi: 10.1111/j.1439-0426.2012.01985.x. Epub 2012 May 21. J Appl Ichthyol. 2012. PMID: 22736877 Free PMC article.
References
-
- Waskiewicz AJ, Rikhof HA, Hernandez RE, Moens CB. Zebrafish Meis functions to stabilize Pbx proteins and regulate hindbrain patterning. Development. 2001;128:4139–4151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

