Modulation of microglial/macrophage activation by macrophage inhibitory factor (TKP) or tuftsin (TKPR) attenuates the disease course of experimental autoimmune encephalomyelitis
- PMID: 17634104
- PMCID: PMC1937009
- DOI: 10.1186/1471-2172-8-10
Modulation of microglial/macrophage activation by macrophage inhibitory factor (TKP) or tuftsin (TKPR) attenuates the disease course of experimental autoimmune encephalomyelitis
Abstract
Background: Myelin Oligodendrocyte Glycoprotein (MOG)-induced experimental autoimmune encephalomyelitis (EAE) is the most commonly used mouse model for multiple sclerosis (MS). During the of progression of EAE, microglia, the immunocompetent cells of the brain, become activated and accumulate around demyelinated lesions. Microglial activation is mediated by the extracellular protease tissue Plasminogen Activator (tPA), and mice lacking tPA display altered EAE progression. In this study, we have used pharmacological inhibitors and stimulators of microglial/macrophage activation to examine the temporal requirement for microglial activation in EAE progression and to determine whether such approaches might potentially be of therapeutic value.
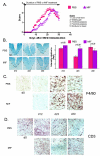
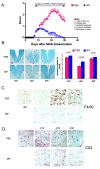
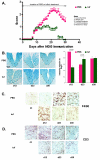
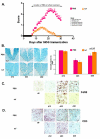
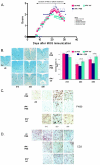
Results: Intervention using the tripeptide macrophage/microglia inhibitory factor MIF (TKP) and the tetrapeptide macrophage/microglial stimulator tuftsin (TKPR) attenuated EAE symptoms and revealed that the timing of macrophage/microglial activation is critical for the clinical outcome of EAE. We show that the disease progression can potentially be manipulated favorably at early stages by altering the timing of microglial activation, which in turn alters the systemic immune response to favor upregulation of T helper cell 2 genes that promote recovery from EAE.
Conclusion: Preventative and therapeutic modulation of macrophage/microglial activity significantly alters the outcome of EAE at symptomatic stages. Specific molecular targets have been identified that represent potential avenues of exploration for the treatment and prevention of MS.
Figures

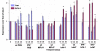
References
-
- Olivares-Villagómez D, Wensky AK, Wang Y, Lafaille JJ. Repertoire Requirements of CD4+ T Cells That Prevent Spontaneous Autoimmune Encephalomyelitis. J Immunol. 2000;164:5499–5507. - PubMed
-
- Chen X, Oppenheim JJ, Winkler-Pickett RT, Ortaldo JR, Howard OM. Glucocorticoid amplifies IL-2-dependent expansion of functional FoxP3(+)CD4(+)CD25(+) T regulatory cells in vivo and enhances their capacity to suppress EAE. Eur J Immunol. 2006;36(8):2139-49:2139–2149. doi: 10.1002/eji.200635873. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

