The zinc finger domain of Wilms' tumor 1 suppressor gene (WT1) behaves as a dominant negative, leading to abrogation of WT1 oncogenic potential in breast cancer cells
- PMID: 17634147
- PMCID: PMC2206716
- DOI: 10.1186/bcr1743
The zinc finger domain of Wilms' tumor 1 suppressor gene (WT1) behaves as a dominant negative, leading to abrogation of WT1 oncogenic potential in breast cancer cells
Abstract
Introduction: There is growing evidence that the Wilms' tumor 1 suppressor gene (WT1) behaves as an oncogene in some forms of breast cancer. Previous studies have demonstrated that the N-terminal domain of WT1 can act as a dominant negative through self-association. In the studies presented here we have explored the potential for the zinc finger domain (ZF) of WT1 to also have dominant-negative effects, and thus further our understanding of this protein.
Methods: Using full-length and ZF-only forms of WT1 we assessed their effect on the WT1 and c-myc promoter using luciferase and chromatin immunoprecipitation assays. The gene expression levels were determined by quantitative real-time RT-PCR, northern blot and western blot. We also assessed the effect of the ZF-only form on the growth of breast cancer cell lines in culture.
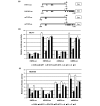
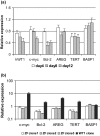
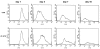
Results: Transfection with WT1-ZF plasmids resulted in a stronger inhibition of WT1 promoter than full-length WT1 in breast cancer cells. The WT1-ZF form lacking the lysine-threonine-serine (KTS) insert (ZF - KTS) can bind to the majority of WT1 consensus sites throughout the WT1 promoter region, while the ZF containing the insert (ZF + KTS) form only binds to sites in the proximal promoter. The abundances of endogenous WT1 mRNA and protein were markedly decreased following the stable expression of ZF - KTS in breast cancer cells. The expressions of WT1 target genes, including c-myc, Bcl-2, amphiregulin and TERT, were similarly suppressed by ZF - KTS. Moreover, WT1-ZF - KTS abrogated the transcriptional activation of c-myc mediated by all four predominant isoforms of WT1 (including or lacking alternatively spliced exons 5 and 9). Finally, WT1-ZF - KTS inhibited colony formation and cell division, but induced apoptosis in MCF-7 cells.
Conclusion: Our observations strongly argue that the WT1-ZF plasmid behaves as a dominant-negative regulator of the endogenous WT1 in breast cancer cells. The inhibition on proliferation of breast cancer cells by WT1-ZF - KTS provides a potential candidate of gene therapy for breast cancer.
Figures

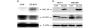


References
-
- Park S, Tomlinson G, Nisen P, Haber DA. Altered trans-activational properties of a mutated WT1 gene product in a WAGR-associated Wilms' tumor. Cancer Res. 1993;53:4757–4760. - PubMed
-
- Bruening W, Gros P, Sato T, Stanimir J, Nakamura Y, Housman D, Pelletier J. Analysis of the 11p13 Wilms' tumor suppressor gene (WT1) in ovarian tumors. Cancer Invest. 1993;11:393–399. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

