Host cell cathepsins potentiate Moloney murine leukemia virus infection
- PMID: 17634228
- PMCID: PMC2045468
- DOI: 10.1128/JVI.02853-06
Host cell cathepsins potentiate Moloney murine leukemia virus infection
Abstract
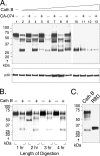
The roles of cellular proteases in Moloney murine leukemia virus (MLV) infection were investigated using MLV particles pseudotyped with vesicular stomatitis virus (VSV) G glycoprotein as a control for effects on core MLV particles versus effects specific to Moloney MLV envelope protein (Env). The broad-spectrum inhibitors cathepsin inhibitor III and E-64d gave comparable dose-dependent inhibition of Moloney MLV Env and VSV G pseudotypes, suggesting that the decrease did not involve the envelope protein. Whereas, CA-074 Me gave a biphasic response that differentiated between Moloney MLV Env and VSV G at low concentrations, at which the drug is highly selective for cathepsin B, but was similar for both glycoproteins at higher concentrations, at which CA-074 Me inhibits other cathepsins. Moloney MLV infection was lower on cathepsin B knockout fibroblasts than wild-type cells, whereas VSV G infection was not reduced on the B-/- cells. Taken together, these results support the notion that cathepsin B acts at an envelope-dependent step while another cathepsin acts at an envelope-independent step, such as uncoating or viral-DNA synthesis. Virus binding was not affected by CA-074 Me, whereas syncytium induction was inhibited in a dose-dependent manner, consistent with cathepsin B involvement in membrane fusion. Western blot analysis revealed specific cathepsin B cleavage of SU in vitro, while TM and CA remained intact. Infection could be enhanced by preincubation of Moloney MLV with cathepsin B, consistent with SU cleavage potentiating infection. These data suggested that during infection of NIH 3T3 cells, endocytosis brings Moloney MLV to early lysosomes, where the virus encounters cellular proteases, including cathepsin B, that cleave SU.
Figures





Similar articles
-
Cathepsin L is required for ecotropic murine leukemia virus infection in NIH3T3 cells.Virology. 2009 Nov 25;394(2):227-34. doi: 10.1016/j.virol.2009.08.045. Epub 2009 Sep 24. Virology. 2009. PMID: 19781728 Free PMC article.
-
Infection of XC cells by MLVs and Ebola virus is endosome-dependent but acidification-independent.PLoS One. 2011;6(10):e26180. doi: 10.1371/journal.pone.0026180. Epub 2011 Oct 12. PLoS One. 2011. PMID: 22022555 Free PMC article.
-
Characterization of R peptide of murine leukemia virus envelope glycoproteins in syncytium formation and entry.Arch Virol. 2007;152(12):2169-82. doi: 10.1007/s00705-007-1054-6. Epub 2007 Sep 14. Arch Virol. 2007. PMID: 17851730
-
Cathepsin B Protease Facilitates Chikungunya Virus Envelope Protein-Mediated Infection via Endocytosis or Macropinocytosis.Viruses. 2020 Jul 3;12(7):722. doi: 10.3390/v12070722. Viruses. 2020. PMID: 32635194 Free PMC article.
-
Selective targeting of lysosomal cysteine proteases with radiolabeled electrophilic substrate analogs.Chem Biol. 2000 Jan;7(1):27-38. doi: 10.1016/s1074-5521(00)00061-2. Chem Biol. 2000. PMID: 10662686
Cited by
-
Critical role of leucine-valine change in distinct low pH requirements for membrane fusion between two related retrovirus envelopes.J Biol Chem. 2012 Mar 2;287(10):7640-51. doi: 10.1074/jbc.M111.334722. Epub 2012 Jan 10. J Biol Chem. 2012. PMID: 22235118 Free PMC article.
-
Retrovirus entry by endocytosis and cathepsin proteases.Adv Virol. 2012;2012:640894. doi: 10.1155/2012/640894. Epub 2012 Dec 6. Adv Virol. 2012. PMID: 23304142 Free PMC article.
-
Cathepsin L is required for ecotropic murine leukemia virus infection in NIH3T3 cells.Virology. 2009 Nov 25;394(2):227-34. doi: 10.1016/j.virol.2009.08.045. Epub 2009 Sep 24. Virology. 2009. PMID: 19781728 Free PMC article.
-
Mouse mammary tumor virus uses mouse but not human transferrin receptor 1 to reach a low pH compartment and infect cells.Virology. 2008 Nov 25;381(2):230-40. doi: 10.1016/j.virol.2008.08.013. Epub 2008 Oct 1. Virology. 2008. PMID: 18829060 Free PMC article.
-
Fragments of Target Cells are Internalized into Retroviral Envelope Protein-Expressing Cells during Cell-Cell Fusion by Endocytosis.Front Microbiol. 2016 Jan 19;6:1552. doi: 10.3389/fmicb.2015.01552. eCollection 2015. Front Microbiol. 2016. PMID: 26834711 Free PMC article.
References
-
- Andersen, K. B. 1987. Cleavage fragments of the retrovirus surface protein gp70 during virus entry. J. Gen. Virol. 68:2193-2202. - PubMed
-
- Andersen, K. B., and B. A. Nexo. 1983. Entry of murine retrovirus into mouse fibroblasts. Virology 125:85-98. - PubMed
-
- Andersen, K. B., and H. Skov. 1989. Retrovirus-induced cell fusion is enhanced by protease treatment. J. Gen. Virol. 70:1921-1927. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

