Enzymes of the taurine biosynthetic pathway are expressed in rat mammary gland
- PMID: 17634260
- PMCID: PMC2099301
- DOI: 10.1093/jn/137.8.1887
Enzymes of the taurine biosynthetic pathway are expressed in rat mammary gland
Abstract
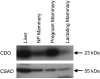
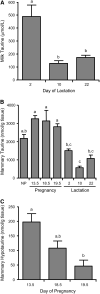
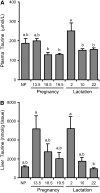
Taurine is the most abundant free amino acid in the body and is present at high concentrations during development and in the early milk. It is synthesized from cysteine via oxidation of cysteine to cysteinesulfinate by the enzyme cysteine dioxygenase (CDO), followed by the decarboxylation of cysteinesulfinate to hypotaurine, catalyzed by cysteine sulfinic acid decarboxylase (CSAD). To determine whether the taurine biosynthetic pathway is present in mammary gland and whether it is differentially expressed during pregnancy and lactation, and also to further explore the possible regulation of hepatic taurine synthesis during pregnancy and lactation, we measured mammary and hepatic CDO and CSAD mRNA and protein concentrations and tissue, plasma and milk taurine concentrations. CDO and CSAD mRNA and protein were expressed in mammary gland and liver regardless of physiological state. Immunohistochemistry demonstrated the expression of CDO in ductal cells of pregnant rats, but not in other mammary epithelial cells or in ductal cells of nonpregnant rats. CDO was also present in stromal adipocytes in mammary glands of both pregnant and nonpregnant rats. Our findings support an upregulation of taurine synthetic capacity in the mammary gland of pregnant rats, based on mammary taurine and hypotaurine concentrations and the intense immunohistochemical staining for CDO in ductal cells of pregnant rats. Hepatic taurine synthetic capacity, particularly CSAD, and taurine concentrations were highest in rats during the early stages of lactation, suggesting the liver may also play a role in the synthesis of taurine to support lactation or repletion of maternal reserves.
Figures



Similar articles
-
3T3-L1 adipocytes and rat adipose tissue have a high capacity for taurine synthesis by the cysteine dioxygenase/cysteinesulfinate decarboxylase and cysteamine dioxygenase pathways.J Nutr. 2009 Feb;139(2):207-14. doi: 10.3945/jn.108.099085. Epub 2008 Dec 23. J Nutr. 2009. PMID: 19106324 Free PMC article.
-
Estradiol decreases taurine level by reducing cysteine sulfinic acid decarboxylase via the estrogen receptor-α in female mice liver.Am J Physiol Gastrointest Liver Physiol. 2015 Feb 15;308(4):G277-86. doi: 10.1152/ajpgi.00107.2014. Epub 2014 Nov 13. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25394658
-
Extrahepatic tissues compensate for loss of hepatic taurine synthesis in mice with liver-specific knockout of cysteine dioxygenase.Am J Physiol Endocrinol Metab. 2012 May 1;302(10):E1292-9. doi: 10.1152/ajpendo.00589.2011. Epub 2012 Mar 13. Am J Physiol Endocrinol Metab. 2012. PMID: 22414809 Free PMC article.
-
Understanding human thiol dioxygenase enzymes: structure to function, and biology to pathology.Int J Exp Pathol. 2017 Apr;98(2):52-66. doi: 10.1111/iep.12222. Epub 2017 Apr 24. Int J Exp Pathol. 2017. PMID: 28439920 Free PMC article. Review.
-
Taurine: new implications for an old amino acid.FEMS Microbiol Lett. 2003 Sep 26;226(2):195-202. doi: 10.1016/S0378-1097(03)00611-6. FEMS Microbiol Lett. 2003. PMID: 14553911 Review.
Cited by
-
The Maternal Milk Microbiome in Mammals of Different Types and Its Potential Role in the Neonatal Gut Microbiota Composition.Animals (Basel). 2021 Nov 23;11(12):3349. doi: 10.3390/ani11123349. Animals (Basel). 2021. PMID: 34944125 Free PMC article.
-
Cysteine dioxygenase: a robust system for regulation of cellular cysteine levels.Amino Acids. 2009 May;37(1):55-63. doi: 10.1007/s00726-008-0202-y. Epub 2008 Nov 15. Amino Acids. 2009. PMID: 19011731 Free PMC article. Review.
-
Ethanol- and/or Taurine-Induced Oxidative Stress in Chick Embryos.J Amino Acids. 2013;2013:240537. doi: 10.1155/2013/240537. Epub 2013 Mar 21. J Amino Acids. 2013. PMID: 23606945 Free PMC article.
-
Arsenic causing gallbladder cancer disease in Bihar.Sci Rep. 2023 Mar 14;13(1):4259. doi: 10.1038/s41598-023-30898-0. Sci Rep. 2023. PMID: 36918592 Free PMC article.
-
Taurine prevents high glucose-induced angiopoietin-2/tie-2 system alterations and apoptosis in retinal microvascular pericytes.Mol Cell Biochem. 2014 Nov;396(1-2):239-48. doi: 10.1007/s11010-014-2159-3. Epub 2014 Jul 25. Mol Cell Biochem. 2014. PMID: 25060907
References
-
- Coloso RM, Hirschberger LL, Dominy JE, Jr., Lee JI, Stipanuk MH. Cysteamine dioxygenase: Evidence for the physiological conversion of cysteamine to hypotaurine in rat and mouse tissues. In: Oja SS, Saransaari P, editors. Taurine 6. Springer; New York: 2006. pp. 25–36. - PubMed
-
- Garcia RA, Stipanuk MH. The splanchnic organs, liver and kidney have unique roles in the metabolism of sulfur amino acids and their metabolites in rats. J Nutr. 1992;122:1693–701. - PubMed
-
- Stipanuk MH. Role of the liver in regulation of body cysteine and taurine levels: a brief review. Neurochem Res. 2004;29:105–10. - PubMed
-
- Stipanuk MH, Londono M, Lee JI, Hu M, Yu AF. Enzymes and metabolites of cysteine metabolism in nonhepatic tissues of rats show little response to changes in dietary protein or sulfur amino acid levels. J Nutr. 2002;132:3369–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

