ADD66, a gene involved in the endoplasmic reticulum-associated degradation of alpha-1-antitrypsin-Z in yeast, facilitates proteasome activity and assembly
- PMID: 17634286
- PMCID: PMC1995736
- DOI: 10.1091/mbc.e07-01-0034
ADD66, a gene involved in the endoplasmic reticulum-associated degradation of alpha-1-antitrypsin-Z in yeast, facilitates proteasome activity and assembly
Abstract
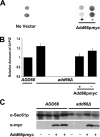
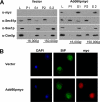
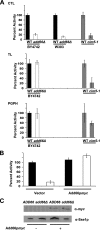
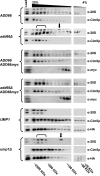
Antitrypsin deficiency is a primary cause of juvenile liver disease, and it arises from expression of the "Z" variant of the alpha-1 protease inhibitor (A1Pi). Whereas A1Pi is secreted from the liver, A1PiZ is retrotranslocated from the endoplasmic reticulum (ER) and degraded by the proteasome, an event that may offset liver damage. To better define the mechanism of A1PiZ degradation, a yeast expression system was developed previously, and a gene, ADD66, was identified that facilitates A1PiZ turnover. We report here that ADD66 encodes an approximately 30-kDa soluble, cytosolic protein and that the chymotrypsin-like activity of the proteasome is reduced in add66Delta mutants. This reduction in activity may arise from the accumulation of 20S proteasome assembly intermediates or from qualitative differences in assembled proteasomes. Add66p also seems to be a proteasome substrate. Consistent with its role in ER-associated degradation (ERAD), synthetic interactions are observed between the genes encoding Add66p and Ire1p, a transducer of the unfolded protein response, and yeast deleted for both ADD66 and/or IRE1 accumulate polyubiquitinated proteins. These data identify Add66p as a proteasome assembly chaperone (PAC), and they provide the first link between PAC activity and ERAD.
Figures

References
-
- Adams A., Gottschling D. E., Kaiser C. A., Stearns T. Plainview, NY: Cold Spring Harbor Laboratory Press; 1997. Methods in Yeast Genetics.
-
- Aridor M., Hannan L. A. Traffic jam: a compendium of human diseases that affect intracellular transport processes. Traffic. 2000;1:836–851. - PubMed
-
- Aridor M., Hannan L. A. Traffic jams II: an update of diseases of intracellular transport. Traffic. 2002;3:781–790. - PubMed
-
- Babbitt S. E., et al. ATP hydrolysis-dependent disassembly of the 26S proteasome is part of the catalytic cycle. Cell. 2005;121:553–565. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

