A unique element in the cytoplasmic tail of the type II transforming growth factor-beta receptor controls basolateral delivery
- PMID: 17634290
- PMCID: PMC1995729
- DOI: 10.1091/mbc.e06-10-0930
A unique element in the cytoplasmic tail of the type II transforming growth factor-beta receptor controls basolateral delivery
Abstract
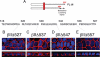
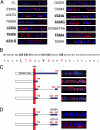
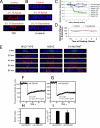
Transforming growth factor (TGF)-beta receptors stimulate diverse signaling processes that control a wide range of biological responses. In polarized epithelia, the TGFbeta type II receptor (T2R) is localized at the basolateral membranes. Sequential cytoplasmic truncations resulted in receptor missorting to apical surfaces, and they indicated an essential targeting element(s) near the receptor's C terminus. Point mutations in the full-length receptor confirmed this prediction, and a unique basolateral-targeting region was elucidated between residues 529 and 538 (LTAxxVAxxR) that was distinct, but colocalized within a clinically significant signaling domain essential for TGFbeta-dependent activation of the Smad2/3 cascade. Transfer of a terminal 84 amino-acid fragment, containing the LTAxxVAxxR element, to the apically sorted influenza hemagglutinin (HA) protein was dominant and directed basolateral HA expression. Although delivery to the basolateral surfaces was direct and independent of any detectable transient apical localization, fluorescence recovery after photobleaching demonstrated similar mobility for the wild-type receptor and a missorted mutant lacking the targeting motif. This latter finding excludes the possibility that the domain acts as a cell membrane retention signal, and it supports the hypothesis that T2R sorting occurs from an intracellular compartment.
Figures




References
-
- Anders R. A., Dore J. J., Jr, Arline S. L., Garamszegi N., Leof E. B. Differential requirement for type I and type II transforming growth factor β receptor kinase activity in ligand-mediated receptor endocytosis. J. Biol. Chem. 1998;273:23118–23125. - PubMed
-
- Anders R. A., Leof E. B. Chimeric granulocyte/macrophage colony-stimulating factor/transforming growth factor-beta (TGF-β) receptors define a model system for investigating the role of homomeric and heteromeric receptors in TGF-β signaling. J. Biol. Chem. 1996;271:21758–21766. - PubMed
-
- Aroeti B., Okhrimenko H., Reich V., Orzech E. Polarized trafficking of plasma membrane proteins: emerging roles for coats, SNAREs, GTPases and their link to the cytoskeleton. Biochim. Biophys. Acta. 1998;1376:57–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

