Chlamydomonas outer arm dynein alters conformation in response to Ca2+
- PMID: 17634291
- PMCID: PMC1951773
- DOI: 10.1091/mbc.e06-10-0917
Chlamydomonas outer arm dynein alters conformation in response to Ca2+
Abstract
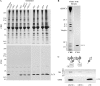
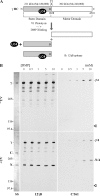
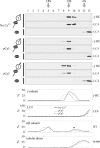
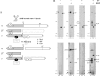
We have previously shown that Ca(2+) directly activates ATP-sensitive microtubule binding by a Chlamydomonas outer arm dynein subparticle containing the beta and gamma heavy chains (HCs). The gamma HC-associated LC4 light chain is a member of the calmodulin family and binds 1-2 Ca(2+) with K(Ca) = 3 x 10(-5) M in vitro, suggesting it may act as a Ca(2+) sensor for outer arm dynein. Here we investigate interactions between the LC4 light chain and gamma HC. Two IQ consensus motifs for binding calmodulin-like proteins are located within the stem domain of the gamma heavy chain. In vitro experiments indicate that LC4 undergoes a Ca(2+)-dependent interaction with the IQ motif domain while remaining tethered to the HC. LC4 also moves into close proximity of the intermediate chain IC1 in the presence of Ca(2+). The sedimentation profile of the gamma HC subunit changed subtly upon Ca(2+) addition, suggesting that the entire complex had become more compact, and electron microscopy of the isolated gamma subunit revealed a distinct alteration in conformation of the N-terminal stem in response to Ca(2+) addition. We propose that Ca(2+)-dependent conformational change of LC4 has a direct effect on the stem domain of the gamma HC, which eventually leads to alterations in mechanochemical interactions between microtubules and the motor domain(s) of the outer dynein arm.
Figures





Similar articles
-
Calcium regulates ATP-sensitive microtubule binding by Chlamydomonas outer arm dynein.J Biol Chem. 2003 Oct 31;278(44):43571-9. doi: 10.1074/jbc.M305894200. Epub 2003 Aug 14. J Biol Chem. 2003. PMID: 12923201
-
Identification of a Ca(2+)-binding light chain within Chlamydomonas outer arm dynein.J Cell Sci. 1995 Dec;108 ( Pt 12):3757-64. doi: 10.1242/jcs.108.12.3757. J Cell Sci. 1995. PMID: 8719882
-
Partially functional outer-arm dynein in a novel Chlamydomonas mutant expressing a truncated gamma heavy chain.Eukaryot Cell. 2008 Jul;7(7):1136-45. doi: 10.1128/EC.00102-08. Epub 2008 May 16. Eukaryot Cell. 2008. PMID: 18487347 Free PMC article.
-
Autoinhibitory and other autoregulatory elements within the dynein motor domain.J Struct Biol. 2006 Oct;156(1):175-81. doi: 10.1016/j.jsb.2006.02.012. Epub 2006 Mar 29. J Struct Biol. 2006. PMID: 16647270 Review.
-
Sensing the mechanical state of the axoneme and integration of Ca2+ signaling by outer arm dynein.Cytoskeleton (Hoboken). 2010 Apr;67(4):207-13. doi: 10.1002/cm.20445. Cytoskeleton (Hoboken). 2010. PMID: 20186692 Free PMC article. Review.
Cited by
-
Calcium sensors of ciliary outer arm dynein: functions and phylogenetic considerations for eukaryotic evolution.Cilia. 2015 Apr 30;4:6. doi: 10.1186/s13630-015-0015-z. eCollection 2015. Cilia. 2015. PMID: 25932323 Free PMC article.
-
Direction of flagellum beat propagation is controlled by proximal/distal outer dynein arm asymmetry.Proc Natl Acad Sci U S A. 2018 Jul 31;115(31):E7341-E7350. doi: 10.1073/pnas.1805827115. Epub 2018 Jul 20. Proc Natl Acad Sci U S A. 2018. PMID: 30030284 Free PMC article.
-
Nucleotide-induced global conformational changes of flagellar dynein arms revealed by in situ analysis.Nat Struct Mol Biol. 2010 Jun;17(6):761-7. doi: 10.1038/nsmb.1832. Epub 2010 May 9. Nat Struct Mol Biol. 2010. PMID: 20453857
-
The flagellar protein Enkurin is required for mouse sperm motility and for transport through the female reproductive tract.Biol Reprod. 2018 Oct 1;99(4):789-797. doi: 10.1093/biolre/ioy105. Biol Reprod. 2018. PMID: 29733335 Free PMC article.
-
Outer-arm dynein light chain LC1 is required for normal motor assembly kinetics, ciliary stability, and motility.Mol Biol Cell. 2023 Jun 1;34(7):ar75. doi: 10.1091/mbc.E23-03-0104. Epub 2023 May 3. Mol Biol Cell. 2023. PMID: 37133971 Free PMC article.
References
-
- Bahler M., Rhoads A. Calmodulin signaling via the IQ motif. FEBS Lett. 2002;513:107–113. - PubMed
-
- Baron D. M., Kabututu Z. P., Hill K. L. Stuck in reverse: loss of LC1 in Trypanosoma brucei disrupts outer dynein arms and leads to reverse flagellar beat and backward movement. J. Cell Sci. 2007;120:1513–1520. - PubMed
-
- Benashski S. E., King S. M. Investigation of protein-protein interactions within flagellar dynein using homobifunctional and zero-length crosslinking reagents. Methods. 2000;22:365–371. - PubMed
-
- Benashski S. E., Patel-King R. S., King S. M. Light chain 1 from the Chlamydomonas outer dynein arm is a leucine-rich repeat protein associated with the motor domain of the γ heavy chain. Biochemistry. 1999;38:7253–7264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

