The amyloid-beta precursor protein is phosphorylated via distinct pathways during differentiation, mitosis, stress, and degeneration
- PMID: 17634293
- PMCID: PMC1995701
- DOI: 10.1091/mbc.e06-07-0625
The amyloid-beta precursor protein is phosphorylated via distinct pathways during differentiation, mitosis, stress, and degeneration
Abstract
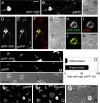
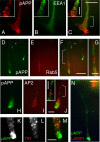
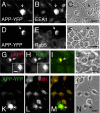
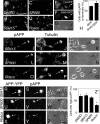
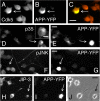
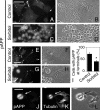
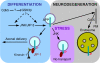
Phosphorylation of amyloid-beta precursor protein (APP) at Thr(668) is a normal process linked to neurite extension and anterograde transport of vesicular cargo. By contrast, increased phosphorylation of APP is a pathological trait of Alzheimer's disease. APP is overexpressed in Down's syndrome, a condition that occasionally leads to increased APP phosphorylation, in cultured cells. Whether phosphorylation of APP in normal versus high APP conditions occurs by similar or distinct signaling pathways is not known. Here, we addressed this problem using brainstem-derived neurons (CAD cells). CAD cells that ectopically overexpress APP frequently show features of degenerating neurons. We found that, in degenerating cells, APP is hyperphosphorylated and colocalizes with early endosomes. By contrast, in normal CAD cells, phosphorylated APP (pAPP) is excluded from endosomes, and localizes to the Golgi apparatus and to transport vesicles within the neurites. Whereas the neuritic APP is phosphorylated by c-Jun NH(2)-terminal kinase through a pathway that is modulated by glycogen synthase kinase 3beta, the endosomal pAPP in degenerated CAD cells results from activation of cyclin-dependent kinase 5. Additional signaling pathways, leading to APP phosphorylation, become active during stress and mitosis. We conclude that distinct pathways of APP phosphorylation operate in proliferating, differentiating, stressed, and degenerating neurons.
Figures

References
-
- Aplin A. E., Gibb G. M., Jacobsen J. S., Gallo J. M., Anderton B. H. In vitro phosphorylation of the cytoplasmic domain of the amyloid precursor protein by glycogen synthase kinase-3beta. J. Neurochem. 1996;67:699–707. - PubMed
-
- Ball C. L., Hunt S. P., Robinson M. S. Expression and localization of alpha-adaptin isoforms. J. Cell Sci. 1995;108:2865–2875. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

