The microtubule-associated protein tumor overexpressed gene/cytoskeleton-associated protein 5 is necessary for myelin basic protein expression in oligodendrocytes
- PMID: 17634360
- PMCID: PMC6672871
- DOI: 10.1523/JNEUROSCI.0203-07.2007
The microtubule-associated protein tumor overexpressed gene/cytoskeleton-associated protein 5 is necessary for myelin basic protein expression in oligodendrocytes
Abstract
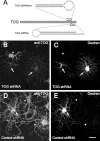
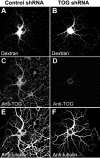
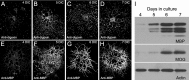
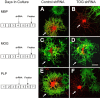
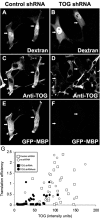
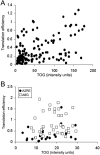
Tumor overexpressed gene (TOG) protein, encoded by cytoskeleton-associated protein CKAP5, is a microtubule-associated protein that binds to heterogeneous nuclear ribonucleoprotein (hnRNP) A2. hnRNP A2 is an RNA trafficking factor that associates with myelin basic protein (MBP) mRNA. In oligodendrocytes, TOG, hnRNP A2, and MBP mRNA colocalize in granules that assemble in the perikaryon and are transported to the peripheral network of processes that extends from it. MBP accumulates preferentially in the membrane of the medial and distal portions of these cellular processes. MBP expression was reduced when TOG level was lowered by short-hairpin (sh) RNA. The reduction in TOG did not affect overall cell morphology or the assembly, transport, localization, or number of MBP mRNA-containing granules. Reduced levels of TOG did not affect another oligodendrocyte-specific component, myelin oligodendrocyte glycoprotein, which is expressed at the same time as MBP but translated from mRNA localized in the cell body. Expression in a neural cell line of a green fluorescent protein (GFP)-MBP fusion protein derived from a construct containing GFP and the full-length cDNA for the rat 14 kDa MBP was reduced when TOG level was lowered by shRNA treatment. Expression of GFP, derived from GFP mRNA containing the hnRNP A2 binding element of MBP mRNA, was similarly reduced in cells with low TOG levels. These data indicate that TOG is necessary for efficient translation of MBP mRNA and suggest that this role is mediated by its interaction with hnRNP A2.
Figures

References
-
- Akhmanova A, Hoogenraad C. Microtubule plus-end-tracking proteins: mechanisms and functions. Curr Opin Cell Biol. 2005;17:47–54. - PubMed
-
- Amur-Umarjee S, Schonmann V, Campagnoni A. Neuronal regulation of myelin basic protein mRNA translocation in oligodendrocytes is mediated by platelet-derived growth factor. Dev Neurosci. 1997;19:143–151. - PubMed
-
- Barbarese E, Brumwell C, Kwon S, Cui H, Carson J. RNA on the road to myelin. J Neurocytol. 1999;28:263–270. - PubMed
-
- Browne G, Proud C. Regulation of peptide-chain elongation in mammalian cells. Eur J Biochem. 2002;269:5360–5368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
