Presynaptic contributions of chordin to hippocampal plasticity and spatial learning
- PMID: 17634368
- PMCID: PMC6672865
- DOI: 10.1523/JNEUROSCI.1604-07.2007
Presynaptic contributions of chordin to hippocampal plasticity and spatial learning
Abstract
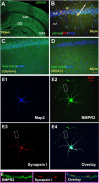
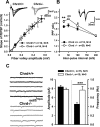
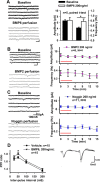
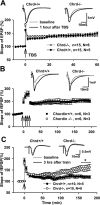
Recently, several evolutionary conserved signaling pathways that play prominent roles in regulating early neurodevelopment have been found to regulate synaptic remodeling in the adult. To test whether adult neuronal expression of bone morphogenic protein (BMP) signaling components also plays a postnatal role in regulating neuronal plasticity, we modulated BMP signaling in mice both in vivo and in vitro by genetic removal of the BMP inhibitor chordin or by perfusing recombinant BMP signaling pathway components onto acute hippocampal slices. Chordin null mice exhibited a significant increase in presynaptic transmitter release from hippocampal neurons, resulting in enhanced paired-pulse facilitation and long-term potentiation. These mice also showed a decreased acquisition time in a water maze test along with less exploratory activity during Y-maze and open-field tests. Perfusion of BMP ligands onto hippocampal slices replicated the presynaptic phenotype of chordin null slices, but bath application of Noggin, another antagonist of BMP signaling pathway, significantly decrease the frequency of miniature EPSCs. These results demonstrate that the BMP signaling pathway contributes to synaptic plasticity and learning likely through a presynaptic mechanism.
Figures



Similar articles
-
Calcium chelation improves spatial learning and synaptic plasticity in aged rats.Exp Neurol. 2006 Feb;197(2):291-300. doi: 10.1016/j.expneurol.2005.06.014. Epub 2005 Jul 22. Exp Neurol. 2006. PMID: 16039651
-
Postsynaptically synthesized prostaglandin E2 (PGE2) modulates hippocampal synaptic transmission via a presynaptic PGE2 EP2 receptor.J Neurosci. 2005 Oct 26;25(43):9858-70. doi: 10.1523/JNEUROSCI.2392-05.2005. J Neurosci. 2005. PMID: 16251433 Free PMC article.
-
Impaired hippocampal synaptic transmission and plasticity in mice lacking fibroblast growth factor 14.Mol Cell Neurosci. 2007 Mar;34(3):366-77. doi: 10.1016/j.mcn.2006.11.020. Epub 2007 Jan 8. Mol Cell Neurosci. 2007. PMID: 17208450
-
Presynaptic release probability is increased in hippocampal neurons from ASIC1 knockout mice.J Neurophysiol. 2008 Feb;99(2):426-41. doi: 10.1152/jn.00940.2007. Epub 2007 Dec 19. J Neurophysiol. 2008. PMID: 18094106
-
Gene targeting of presynaptic proteins in synaptic plasticity and memory: across the great divide.Neurobiol Learn Mem. 2006 Jan;85(1):2-15. doi: 10.1016/j.nlm.2005.08.014. Epub 2005 Oct 14. Neurobiol Learn Mem. 2006. PMID: 16230036 Free PMC article. Review.
Cited by
-
Fly LMBR1/LIMR-type protein Lilipod promotes germ-line stem cell self-renewal by enhancing BMP signaling.Proc Natl Acad Sci U S A. 2015 Nov 10;112(45):13928-33. doi: 10.1073/pnas.1509856112. Epub 2015 Oct 28. Proc Natl Acad Sci U S A. 2015. PMID: 26512105 Free PMC article.
-
Ketamine Protects Gamma Oscillations by Inhibiting Hippocampal LTD.PLoS One. 2016 Jul 28;11(7):e0159192. doi: 10.1371/journal.pone.0159192. eCollection 2016. PLoS One. 2016. PMID: 27467732 Free PMC article.
-
Astrocyte-induced synaptogenesis is mediated by transforming growth factor β signaling through modulation of D-serine levels in cerebral cortex neurons.J Biol Chem. 2012 Nov 30;287(49):41432-45. doi: 10.1074/jbc.M112.380824. Epub 2012 Oct 10. J Biol Chem. 2012. PMID: 23055518 Free PMC article.
-
The expression of twisted gastrulation in postnatal mouse brain and functional implications.Neuroscience. 2010 Aug 25;169(2):920-31. doi: 10.1016/j.neuroscience.2010.05.026. Epub 2010 May 20. Neuroscience. 2010. PMID: 20493240 Free PMC article.
-
Increased BMP6 levels in the brains of Alzheimer's disease patients and APP transgenic mice are accompanied by impaired neurogenesis.J Neurosci. 2010 Sep 15;30(37):12252-62. doi: 10.1523/JNEUROSCI.1305-10.2010. J Neurosci. 2010. PMID: 20844121 Free PMC article.
References
-
- Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. - PubMed
-
- Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhaes TR, Goodman CS. wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila . Neuron. 2002;33:545–558. - PubMed
-
- Bachiller D, Klingensmith J, Kemp C, Belo JA, Anderson RM, May SR, McMahon JA, McMahon AP, Harland RM, Rossant J, De Robertis EM. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature. 2000;403:658–661. - PubMed
-
- Bachiller D, Klingensmith J, Shneyder N, Tran U, Anderson R, Rossant J, De Robertis EM. The role of chordin/Bmp signals in mammalian pharyngeal development and DiGeorge syndrome. Development. 2003;130:3567–3578. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
