Minocycline alleviates death of oligodendrocytes by inhibiting pro-nerve growth factor production in microglia after spinal cord injury
- PMID: 17634369
- PMCID: PMC6672884
- DOI: 10.1523/JNEUROSCI.1661-07.2007
Minocycline alleviates death of oligodendrocytes by inhibiting pro-nerve growth factor production in microglia after spinal cord injury
Abstract
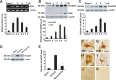
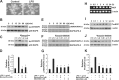
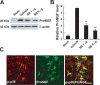
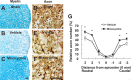
Spinal cord injury (SCI) causes a permanent neurological disability, and no satisfactory treatment is currently available. After SCI, pro-nerve growth factor (proNGF) is known to play a pivotal role in apoptosis of oligodendrocytes, but the cell types producing proNGF and the signaling pathways involved in proNGF production are primarily unknown. Here, we show that minocycline improves functional recovery after SCI in part by reducing apoptosis of oligodendrocytes via inhibition of proNGF production in microglia. After SCI, the stress-responsive p38 mitogen-activated protein kinase (p38MAPK) was activated only in microglia, and proNGF was produced by microglia via the p38MAPK-mediated pathway. Minocycline treatment significantly reduced proNGF production in microglia in vitro and in vivo by inhibition of the phosphorylation of p38MAPK. Furthermore, minocycline treatment inhibited p75 neurotrophin receptor expression and RhoA activation after injury. Finally, minocycline treatment inhibited oligodendrocyte death and improved functional recovery after SCI. These results suggest that minocycline may represent a potential therapeutic agent for acute SCI in humans.
Figures






Similar articles
-
MMP-3 secreted from endothelial cells of blood vessels after spinal cord injury activates microglia, leading to oligodendrocyte cell death.Neurobiol Dis. 2015 Oct;82:141-151. doi: 10.1016/j.nbd.2015.06.002. Epub 2015 Jun 12. Neurobiol Dis. 2015. PMID: 26079709
-
Inhibition of inflammation and oxidative stress by Angelica dahuricae radix extract decreases apoptotic cell death and improves functional recovery after spinal cord injury.J Neurosci Res. 2012 Jan;90(1):243-56. doi: 10.1002/jnr.22734. Epub 2011 Sep 15. J Neurosci Res. 2012. PMID: 21922518
-
17β-Estradiol inhibits apoptotic cell death of oligodendrocytes by inhibiting RhoA-JNK3 activation after spinal cord injury.Endocrinology. 2012 Aug;153(8):3815-27. doi: 10.1210/en.2012-1068. Epub 2012 Jun 14. Endocrinology. 2012. PMID: 22700771
-
Cell death in models of spinal cord injury.Prog Brain Res. 2002;137:37-47. doi: 10.1016/s0079-6123(02)37006-7. Prog Brain Res. 2002. PMID: 12440358 Review.
-
Critical data-based re-evaluation of minocycline as a putative specific microglia inhibitor.Glia. 2016 Oct;64(10):1788-94. doi: 10.1002/glia.23007. Epub 2016 Jun 1. Glia. 2016. PMID: 27246804 Review.
Cited by
-
Electroporation-mediated gene delivery of cleavage-resistant pro-nerve growth factor causes retinal neuro- and vascular degeneration.Mol Vis. 2012;18:2993-3003. Epub 2012 Dec 14. Mol Vis. 2012. PMID: 23288991 Free PMC article.
-
Improving outcomes of neuroprotection by minocycline: guides from cell culture and intracerebral hemorrhage in mice.Am J Pathol. 2010 Mar;176(3):1193-202. doi: 10.2353/ajpath.2010.090361. Epub 2010 Jan 28. Am J Pathol. 2010. PMID: 20110416 Free PMC article.
-
Beyond the lesion site: minocycline augments inflammation and anxiety-like behavior following SCI in rats through action on the gut microbiota.J Neuroinflammation. 2021 Jun 26;18(1):144. doi: 10.1186/s12974-021-02123-0. J Neuroinflammation. 2021. PMID: 34174901 Free PMC article.
-
Panax ginseng Improves Functional Recovery after Contusive Spinal Cord Injury by Regulating the Inflammatory Response in Rats: An In Vivo Study.Evid Based Complement Alternat Med. 2015;2015:817096. doi: 10.1155/2015/817096. Epub 2015 Sep 15. Evid Based Complement Alternat Med. 2015. PMID: 26451158 Free PMC article.
-
Interaction of NG2(+) glial progenitors and microglia/macrophages from the injured spinal cord.Glia. 2010 Mar;58(4):410-22. doi: 10.1002/glia.20932. Glia. 2010. PMID: 19780197 Free PMC article.
References
-
- Ackery A, Robins S, Fehlings MG. Inhibition of Fas-mediated apoptosis through administration of soluble Fas receptor improves functional outcome and reduces posttraumatic axonal degeneration after acute spinal cord injury. J Neurotrauma. 2006;23:603–616. - PubMed
-
- Balentine JD. Pathology of experimental spinal cord trauma. I. The necrotic lesion as a function of vascular injury. Lab Invest. 1978;39:236–253. - PubMed
-
- Barrett GL. The p75 neurotrophin receptor and neuronal apoptosis. Prog Neurobiol. 2000;61:205–292. - PubMed
-
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. - PubMed
-
- Beattie MS, Farooqui AA, Bresnahan JC. Review of current evidence for apoptosis after spinal cord injury. J Neurotrauma. 2000;17:915–925. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
