cAMP response element-binding protein deficiency allows for increased neurogenesis and a rapid onset of antidepressant response
- PMID: 17634380
- PMCID: PMC6672880
- DOI: 10.1523/JNEUROSCI.2051-07.2007
cAMP response element-binding protein deficiency allows for increased neurogenesis and a rapid onset of antidepressant response
Abstract
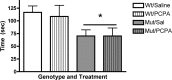
cAMP response element-binding protein (CREB) has been implicated in the molecular and cellular mechanisms of chronic antidepressant (AD) treatment, although its role in the behavioral response is unclear. CREB-deficient (CREB(alpha delta) mutant) mice demonstrate an antidepressant phenotype in the tail suspension test (TST) and forced-swim test. Here, we show that, at baseline, CREB(alpha delta) mutant mice exhibited increased hippocampal cell proliferation and neurogenesis compared with wild-type (WT) controls, effects similar to those observed in WT mice after chronic desipramine (DMI) administration. Neurogenesis was not further augmented by chronic DMI treatment in CREB(alpha delta) mutant mice. Serotonin depletion decreased neurogenesis in CREB(alpha delta) mutant mice to WT levels, which correlated with a reversal of the antidepressant phenotype in the TST. This effect was specific for the reversal of the antidepressant phenotype in these mice, because serotonin depletion did not alter a baseline anxiety-like behavior in CREB(alpha delta) mutant mice. The response to chronic AD treatment in the novelty-induced hypophagia (NIH) test may rely on neurogenesis. Therefore, we used this paradigm to evaluate chronic AD treatment in CREB(alpha delta) mutant mice to determine whether the increased neurogenesis in these mice alters their response in the NIH paradigm. Whereas both WT and CREB(alpha delta) mutant mice responded to chronic AD treatment in the NIH paradigm, only CREB(alpha delta) mutant mice responded to acute AD treatment. However, in the elevated zero maze, DMI did not reverse anxiety behavior in mutant mice. Together, these data show that increased hippocampal neurogenesis allows for an antidepressant phenotype as well as a rapid onset of behavioral responses to AD treatment.
Figures






References
-
- Banasr M, Hery M, Brezun JM, Daszuta A. Serotonin mediates oestrogen stimulation of cell proliferation in the adult dentate gyrus. Eur J Neurosci. 2001;14:1417–1424. - PubMed
-
- Bechtholt AJ, Hill TE, Lucki I. Anxiolytic effect of serotonin depletion in the novelty-induced hypophagia test. Psychopharmacology (Berl) 2007;190:531–540. - PubMed
-
- Blendy JA. The role of CREB in depression and antidepressant treatment. Biol Psychiatry. 2006;59:1144–1150. - PubMed
-
- Blom JM, Tascedda F, Carra S, Ferraguti C, Barden N, Brunello N. Altered regulation of CREB by chronic antidepressant administration in the brain of transgenic mice with impaired glucocorticoid receptor function. Neuropsychopharmacology. 2002;26:605–614. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
