Kidney-specific enhancement of ANG II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice
- PMID: 17634399
- PMCID: PMC2000297
- DOI: 10.1152/ajprenal.00146.2007
Kidney-specific enhancement of ANG II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice
Abstract
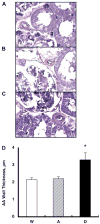
This study was performed in transgenic mice to test the hypothesis that the selective intrarenal overproduction of ANG II increases intrarenal mouse (m) angiotensinogen (AGT) expression. We used the following three groups: 1) single transgenic mice (group A, n = 14) expressing human (h) AGT only in the kidney, 2) double-transgenic mice (group D, n = 13) expressing human renin systemically in addition to hAGT only in the kidney, and 3) wild-type (group W, n = 12) mice. Exogenous hAGT protein is inactive in group A because endogenous mouse renin cannot cleave hAGT to ANG I because of a high species specificity. All mice were monitored from 12 to 18 wk of age. Systolic blood pressure progressively increased from 116 +/- 5 mmHg (12 wk) to 140 +/- 7 (18 wk) in group D. This increase was not observed in groups A or W. Intrarenal hAGT levels were similar in groups A and D; however, hAGT was not detectable in kidneys of group W. Kidney ANG II levels were increased in group D (216 +/- 43 fmol/g) compared with groups A (117 +/- 16) and W (118 +/- 17). However, plasma ANG II concentrations were similar among the three groups. Endogenous renal mAGT mRNA was increased significantly in group D (1.46 +/- 0.19, ratio) compared with groups A (0.97 +/- 0.12) and W (1.00 +/- 0.08). Endogenous renal mAGT protein was also significantly increased in group D compared with groups A and W. Interstitial collagen-positive area, interstitial macrophage/monocyte infiltration, and afferent arteriolar wall thickness were increased significantly in group D compared with groups A and W. These data indicate for the first time that the selective stimulation of intrarenal production of ANG II from hAGT augments endogenous intrarenal mAGT mRNA and protein expression.
Figures



References
-
- Baltatu O, Silva JA, Jr, Ganten D, Bader M. The brain renin-angiotensin system modulates angiotensin II-induced hypertension and cardiac hypertrophy. Hypertension. 2000;35:409–412. - PubMed
-
- Carey AV, Carey RM, Gomez RA. Expression of alpha-smooth muscle actin in the developing kidney vasculature. Hypertension. 1992;19:II168–II175. - PubMed
-
- Casarini DE, Boim MA, Stella RC, Krieger-Azzolini MH, Krieger JE, Schor N. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol Renal Physiol. 1997;272:F405–F409. - PubMed
-
- Danser AH, Admiraal PJ, Derkx FH, Schalekamp MA. Angiotensin I-to-II conversion in the human renal vascular bed. J Hypertens. 1998;16:2051–2056. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

