Feasibility of a multiplex flow cytometric bead immunoassay for detection of anti-epoetin alfa antibodies
- PMID: 17634512
- PMCID: PMC2043303
- DOI: 10.1128/CVI.00157-07
Feasibility of a multiplex flow cytometric bead immunoassay for detection of anti-epoetin alfa antibodies
Abstract
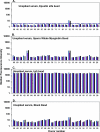
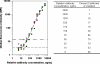
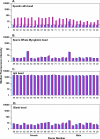
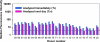
Immunogenicity profiles of recombinant therapeutic proteins are important to understand because antibodies raised against these molecules may have important clinical sequelae. The purpose of the present study was to demonstrate that a flow cytometric bead array could be used to detect clinically relevant antibodies with specificity to such therapeutics. We chose to evaluate well-characterized specimens from persons treated with epoetin alfa that developed antibody-mediated pure red blood cell aplasia as a means to demonstrate the utility of this platform. Our data show that this assay is capable of detecting anti-epoetin alfa antibodies with a relative antibody concentration of 50 ng/ml, where 25 of 25 sera spiked with antibodies at this concentration scored positive. Moreover, the assay was designed to include positive and negative control beads for each specimen that is processed to ensure the specificity of the signal when detected. Measurement of interassay precision supports quantitative estimates of relative antibody concentrations in the range of 313 to 5,000 ng/ml, where the percent coefficient of variation did not exceed 20%. With respect to clinical specimens, antibodies with specificity for epoetin alfa could be easily detected in a set of specimens from persons with pure red blood cell aplasia that had prior exposure to the EPREX brand of recombinant epoetin alfa. Further development and validation of this approach may facilitate successful widespread application of the method for detection of anti-epoetin alfa antibodies, as well as antibodies directed against other recombinant therapeutic proteins.
Figures


References
-
- Bennett, C. L., D. Cournoyer, K. R. Carson, J. Rossert, S. Luminari, A. M. Evens, F. Locatelli, S. M. Belknap, J. M. McKoy, E. A. Lyons, B. Kim, R. Sharma, S. Costello, E. B. Toffelmire, G. A. Wells, H. A. Messner, P. R. Yarnold, S. M. Trifilio, D. W. Raisch, T. M. Kuzel, A. Nissenson, L. C. Lim, M. S. Tallman, and N. Casadevall. 2005. Long-term outcome of individuals with pure red cell aplasia and antierythropoietin antibodies in patients treated with recombinant epoetin: a follow-up report from the Research on Adverse Drug Events and Reports (RADAR) project. Blood 1063343-3347. - PMC - PubMed
-
- Boven, K., J. Knight, F. Bader, J. Rossert, K. U. Eckardt, and N. Casadevall. 2005. Epoetin-associated pure red cell aplasia in patients with chronic kidney disease: solving the mystery. Nephrol. Dialysis Transplant. 20iii33-iii40. - PubMed
-
- Boven, K., S. Stryker, J. Knight, A. Thomas, M. van Regenmortel, D. M. Kemeny, D. Power, J. Rossert, and N. Casadevall. 2005. The increased incidence of pure red cell aplasia with an Eprex formulation in uncoated rubber stopper syringes. Kidney Int. 672346-2353. - PubMed
-
- Casadevall, N., J. Nataf, B. Viron, A. Kolta, J. J. Kiladjian, P. Martin-Dupont, P. Michaud, T. Papo, V. Ugo, I. Teyssandier, B. Varet, and P. Mayeux. 2002. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N. Engl. J. Med. 346469-475. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

