Interaction between midazolam and clarithromycin in the elderly
- PMID: 17635500
- PMCID: PMC2291277
- DOI: 10.1111/j.1365-2125.2007.02970.x
Interaction between midazolam and clarithromycin in the elderly
Abstract
Aim: To assess the relative contribution of intestinal and hepatic CYP3A inhibition to the interaction between the prototypic CYP3A substrate midazolam and clarithromycin in the elderly.
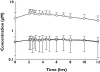
Methods: On day 1, 16 volunteers (eight male, eight female) aged 65-75 years weighing 59-112 kg received simultaneous doses of midazolam intravenously (i.v.) (0.05 mg kg(-1) over 30 min) and orally (p.o.) (3.5 mg of a stable isotope, (15)N(3)-midazolam). Starting on day 2, clarithromycin 500 mg was administered orally twice daily for 7 days. On day eight, i.v. and p.o. doses of midazolam were administered 2 h after the final clarithromycin dose. Serum and urine samples were assayed for midazolam, (15)N(3)-midazolam and metabolites by gas chromatography/mass spectometry.
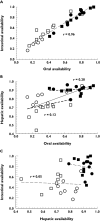
Results: Men and women exhibited similar i.v. (30.4 vs. 36.0 l h(-1)) and p.o. (119 vs. 124 l h(-1)) clearances of midazolam. Midazolam hepatic availability was significantly (P = 0.006) greater in men [0.79, 95% confidence interval (CI) 0.75, 0.84] than in women (0.66, 95% CI 0.59, 0.73), but midazolam intestinal availability (0.39 vs. 0.55) was not different. Following clarithromycin dosing, a significant decrease in systemic (33.2 l h(-1) to 11.5 l h(-1)) and oral (121 l h(-1) to 17.4 l h(-1)) midazolam clearance occurred. Oral, hepatic and intestinal availability was significantly increased after clarithromycin dosing from 0.34 to 0.72, 0.73 to 0.91 and 0.47 to 0.79, respectively. Clarithromycin administration led to an increase in the AUC of midazolam by 3.2-fold following i.v. dosing and 8.0-fold following p.o. dosing. Similar effects were observed for males and females.
Conclusions: Intestinal and hepatic CYP3A inhibition by clarithromycin significantly reduces the clearance of midazolam in the elderly.
Figures


Similar articles
-
The contribution of intestinal and hepatic CYP3A to the interaction between midazolam and clarithromycin.Clin Pharmacol Ther. 1998 Aug;64(2):133-43. doi: 10.1016/S0009-9236(98)90146-1. Clin Pharmacol Ther. 1998. PMID: 9728893
-
The effect of age, sex, and rifampin administration on intestinal and hepatic cytochrome P450 3A activity.Clin Pharmacol Ther. 2003 Sep;74(3):275-87. doi: 10.1016/S0009-9236(03)00187-5. Clin Pharmacol Ther. 2003. PMID: 12966371 Clinical Trial.
-
The effect of hormone replacement therapy on CYP3A activity.Clin Pharmacol Ther. 2000 Oct;68(4):412-7. doi: 10.1067/mcp.2000.110560. Clin Pharmacol Ther. 2000. PMID: 11061581
-
Clinical pharmacokinetics of clarithromycin.Clin Pharmacokinet. 1999 Nov;37(5):385-98. doi: 10.2165/00003088-199937050-00003. Clin Pharmacokinet. 1999. PMID: 10589373 Review.
-
The pharmacokinetics of clarithromycin and its 14-OH metabolite.J Hosp Infect. 1991 Sep;19 Suppl A:29-37. doi: 10.1016/0195-6701(91)90215-t. J Hosp Infect. 1991. PMID: 1684980 Review.
Cited by
-
Volume of Distribution is Unaffected by Metabolic Drug-Drug Interactions.Clin Pharmacokinet. 2021 Feb;60(2):205-222. doi: 10.1007/s40262-020-00926-7. Clin Pharmacokinet. 2021. PMID: 32725383 Free PMC article.
-
Physiologically-based pharmacokinetic modeling to predict CYP3A4-mediated drug-drug interactions of finerenone.CPT Pharmacometrics Syst Pharmacol. 2022 Feb;11(2):199-211. doi: 10.1002/psp4.12746. Epub 2021 Nov 25. CPT Pharmacometrics Syst Pharmacol. 2022. PMID: 34783193 Free PMC article.
-
Effect of rifampicin and clarithromycin on the CYP3A activity in patients with Mycobacterium avium complex.J Thorac Dis. 2019 Sep;11(9):3814-3821. doi: 10.21037/jtd.2019.09.06. J Thorac Dis. 2019. PMID: 31656654 Free PMC article.
-
Concurrent assessment of hepatic and intestinal cytochrome P450 3A activities using deuterated alfentanil.Clin Pharmacol Ther. 2011 Apr;89(4):562-70. doi: 10.1038/clpt.2010.313. Epub 2011 Feb 23. Clin Pharmacol Ther. 2011. PMID: 21346758 Free PMC article. Clinical Trial.
-
Predicting Clinical Effects of CYP3A4 Modulators on Abemaciclib and Active Metabolites Exposure Using Physiologically Based Pharmacokinetic Modeling.J Clin Pharmacol. 2020 Jul;60(7):915-930. doi: 10.1002/jcph.1584. Epub 2020 Feb 20. J Clin Pharmacol. 2020. PMID: 32080863 Free PMC article.
References
-
- Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994;270:414–23. - PubMed
-
- Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, Watkins PB, Daly A, Wrighton SA, Hall SD, Maurel P, Relling M, Brimer C, Yasuda K, Venkataramanan R, Strom S, Thummel K, Boguski MS, Schuetz E. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27:383–91. - PubMed
-
- Roy JN, Lajoie J, Zijenah LS, Barama A, Poirier C, Ward BJ, Roger M. CYP3A5 genetic polymorphisms in different ethnic populations. Drug Metab Dispos. 2005;33:884–7. - PubMed
-
- Zhao Y, Song M, Guan D, Bi S, Meng J, Li Q, Wang W. Genetic polymorphisms of CYP3A5 genes and concentration of the cyclosporine and tacrolimus. Transplant Proc. 2005;37:178–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

