Pharmacokinetics and clinical efficacy of lorazepam in children with severe malaria and convulsions
- PMID: 17635501
- PMCID: PMC2291276
- DOI: 10.1111/j.1365-2125.2007.02966.x
Pharmacokinetics and clinical efficacy of lorazepam in children with severe malaria and convulsions
Abstract
Aim: To investigate the pharmacokinetics and clinical efficacy of intravenous (i.v.) and intramuscular (i.m.) lorazepam (LZP) in children with severe malaria and convulsions.
Methods: Twenty-six children with severe malaria and convulsions lasting > or =5 min were studied. Fifteen children were given a single dose (0.1 mg kg(-1)) of i.v. LZP and 11 received a similar i.m. dose. Blood samples were collected over 72 h for determination of plasma LZP concentrations. Plasma LZP concentration-time data were fitted using compartmental models.
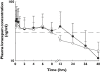
Results: Median [95% confidence interval (CI)] LZP concentrations of 65.1 ng ml(-1) (50.2, 107.0) and 41.4 ng ml(-1) (22.0, 103.0) were attained within median (95% CI) times of 30 min (10, 40) and 25 min (20, 60) following i.v. and i.m. administration, respectively. Concentrations were maintained above the reported therapeutic concentration (30 ng ml(-1)) for at least 8 h after dosing via either route. The relative bioavailability of i.m. LZP was 89%. A single dose of LZP was effective for rapid termination of convulsions in all children and prevention of seizure recurrence for >72 h in 11 of 15 children (73%, i.v.) and 10 of 11 children (91%, i.m), without any clinically apparent respiratory depression or hypotension. Three children (12%) died.
Conclusion: Administration of LZP (0.1 mg kg(-1)) resulted in rapid achievement of plasma LZP concentrations within the reported effective therapeutic range without significant cardiorespiratory effects. I.m administration of LZP may be more practical in rural healthcare facilities in Africa, where venous access may not be feasible.
Figures
Similar articles
-
Pharmacokinetics and clinical efficacy of midazolam in children with severe malaria and convulsions.Br J Clin Pharmacol. 2008 Oct;66(4):529-38. doi: 10.1111/j.1365-2125.2008.03239.x. Epub 2008 Jun 9. Br J Clin Pharmacol. 2008. PMID: 18662297 Free PMC article. Clinical Trial.
-
Determination of lorazepam in plasma from children by high-performance liquid chromatography with UV detection.J Chromatogr B Analyt Technol Biomed Life Sci. 2005 Sep 25;824(1-2):333-40. doi: 10.1016/j.jchromb.2005.07.040. J Chromatogr B Analyt Technol Biomed Life Sci. 2005. PMID: 16112623
-
Pharmacokinetics and anticonvulsant effects of diazepam in children with severe falciparum malaria and convulsions.Br J Clin Pharmacol. 2002 Jan;53(1):49-57. doi: 10.1046/j.0306-5251.2001.01529.x. Br J Clin Pharmacol. 2002. PMID: 11849195 Free PMC article.
-
Intramuscular and rectal therapies of acute seizures.Epilepsy Behav. 2015 Aug;49:307-12. doi: 10.1016/j.yebeh.2015.05.001. Epub 2015 Jun 11. Epilepsy Behav. 2015. PMID: 26071998 Review.
-
Best BETs from the Manchester Royal Infirmary. BET 1: intranasal lorazepam is an acceptable alternative to intravenous lorazepam in the control of acute seizures in children.Emerg Med J. 2013 Sep;30(9):768-9. doi: 10.1136/emermed-2013-202981.1. Emerg Med J. 2013. PMID: 23943640 Review.
Cited by
-
[Epilepsy and acute seizures in childhood in sub-Saharan Africa: challenges and hopes].Pan Afr Med J. 2016 Feb 29;23:58. doi: 10.11604/pamj.2016.23.58.3273. eCollection 2016. Pan Afr Med J. 2016. PMID: 27217883 Free PMC article. Review. French.
-
Treatment of community-onset, childhood convulsive status epilepticus: a prospective, population-based study.Lancet Neurol. 2008 Aug;7(8):696-703. doi: 10.1016/S1474-4422(08)70141-8. Epub 2008 Jul 2. Lancet Neurol. 2008. PMID: 18602345 Free PMC article. Clinical Trial.
-
Risk factors associated with death in in-hospital pediatric convulsive status epilepticus.PLoS One. 2012;7(10):e47474. doi: 10.1371/journal.pone.0047474. Epub 2012 Oct 26. PLoS One. 2012. PMID: 23110074 Free PMC article.
-
Population Pharmacokinetics and Exploratory Pharmacodynamics of Lorazepam in Pediatric Status Epilepticus.Clin Pharmacokinet. 2017 Aug;56(8):941-951. doi: 10.1007/s40262-016-0486-0. Clin Pharmacokinet. 2017. PMID: 27943220 Free PMC article. Clinical Trial.
References
-
- Crawley J, Smith S, Kirkham F, Muthinji P, Waruiru C, Marsh K. Seizures and status epilepticus in childhood cerebral malaria. Q J Med. 1996;89:591–7. - PubMed
-
- Crawley J, Waruiru C, Mithwani S, Mwangi I, Watkins W, Ouma D, Winstanley P, Peto T, Marsh K. Effect of phenobarbital on seizure frequency and mortality in childhood cerebral malaria: a randomised, controlled intervention study. Lancet. 2000;355:701–6. - PubMed
-
- Waruiru CM, Newton CR, Forster D, New L, Winstanley P, Mwangi I, Marsh V, Winstanley M, Snow RW, Marsh K. Epileptic seizures and malaria in Kenyan children. Trans R Soc Trop Med Hyg. 1996;90:152–5. - PubMed
-
- Waller D, Krishna S, Crawley J, Miller K, Nosten F, Chapman D, ter Kuile FO, Craddock C, Berry C, Holloway PA, Brewster D, Greenwood BM, White NJ. Clinical features and outcome of severe malaria in Gambian children. Clin Infect Dis. 1995;21:577–87. - PubMed
-
- Holding PA, Stevenson J, Peshu N, Marsh K. Cognitive sequelae of severe malaria with impaired consciousness. Trans R Soc Trop Med Hyg. 1999;93:529–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

