Neuron-glia signaling in trigeminal ganglion: implications for migraine pathology
- PMID: 17635592
- PMCID: PMC2268711
- DOI: 10.1111/j.1526-4610.2007.00854.x
Neuron-glia signaling in trigeminal ganglion: implications for migraine pathology
Abstract
Objective: The goal of this study was to investigate neuronal-glial cell signaling in trigeminal ganglia under basal and inflammatory conditions using an in vivo model of trigeminal nerve activation.
Background: Activation of trigeminal ganglion nerves and release of calcitonin gene-related peptide (CGRP) are implicated in the pathology of migraine. Cell bodies of trigeminal neurons reside in the ganglion in close association with glial cells. Neuron-glia interactions are involved in all stages of inflammation and pain associated with several central nervous system (CNS) diseases. However, the role of neuron-glia interactions within the trigeminal ganglion under normal and inflammatory conditions is not known.
Methods: Sprague-Dawley rats were utilized to study neuron-glia signaling in the trigeminal ganglion. Initially, True Blue was used as a retrograde tracer to localize neuronal cell bodies in the ganglion by fluorescent microscopy and multiple image alignment. Dye-coupling studies were conducted under basal conditions and in response to capsaicin injection into the TMJ capsule. S100B and p38 expression in neurons and glia were determined by immunohistochemistry following chemical stimulation. CGRP levels in the ganglion were measured by radioimmunoassay in response to capsaicin. In addition, the effect of CGRP on the release of 19 different cytokines from cultured glial cells was investigated by protein microarray analysis.
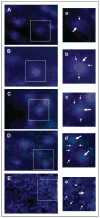
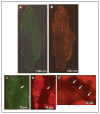
Results: In unstimulated control animals, True Blue was detected primarily in neuronal cell bodies localized in clusters within the ganglion corresponding to the V3 region (TMJ capsule), V2 region (whisker pad), or V1 region (eyebrow and eye). However, True Blue was detected in both neuronal cell bodies and adjacent glia in the V3 region of the ganglion obtained from animals injected with capsaicin. Dye movement into the surrounding glia correlated with the time after capsaicin injection. Chemical stimulation of V3 trigeminal nerves was found to increase the expression of the inflammatory proteins S100B and p38 in both neurons and glia within the V3 region. Unexpectedly, increased levels of these proteins were also observed in the V2 and V1 regions of the ganglion. CGRP and the vesicle docking protein SNAP-25 were colocalized in many neuronal cell bodies and processes. Decreased CGRP levels in the ganglion were observed 2 hours following capsaicin stimulation. Using protein microarray analysis, CGRP was shown to differentially regulate cytokine secretion from cultured trigeminal ganglion glia.
Conclusions: We demonstrated that activation of trigeminal neurons leads to changes in adjacent glia that involve communication through gap junctions and paracrine signaling. This is the first evidence, to our knowledge, of neuron-glia signaling via gap junctions within the trigeminal ganglion. Based on our findings, it is likely that neuronal-glial communication via gap junctions and paracrine signaling are involved in the development of peripheral sensitization within the trigeminal ganglion and, thus, are likely to play an important role in the initiation of migraine. Furthermore, we propose that propagation of inflammatory signals within the ganglion may help to explain commonly reported symptoms of comorbid conditions associated with migraine.
Conflict of interest statement
Figures









Comment in
-
Neuron-glia signaling in the trigeminal ganglion.Headache. 2008 Feb;48(2):299-300. doi: 10.1111/j.1526-4610.2007.01013.x. Epub 2008 Jan 7. Headache. 2008. PMID: 18194293 No abstract available.
References
-
- Stewart W, Lipton R, Celentano D, Reed M. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA. 1991;267:64–69. - PubMed
-
- Pietrobon D. Migraine: New molecular mechanisms. Neuroscientist. 2005;11:373–386. - PubMed
-
- Appelgren A, Appelgren B, Kopp S, Lundeberg T, Theodorsson E. Neuropeptides in the arthritic TMJ and symptoms and signs from the stomatognathic system with special considerations to rheumatoid arthritis. J Orofac Pain. 1995;9:215–225. - PubMed
-
- Lazarov N. Comparative analysis of the chemical neuroanatomy of the mammalian trigeminal ganglion and mesencephalic trigeminal nucleus. Prog Neurobiol. 2002;66:19–59. - PubMed
-
- Hargreaves R, Shepheard S. Pathophysiology of migraine—New insights. Can J Neurol Sci. 1999;26:S12–S19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

