Interleukin-21 differentially affects human natural killer cell subsets
- PMID: 17635612
- PMCID: PMC2266033
- DOI: 10.1111/j.1365-2567.2007.02675.x
Interleukin-21 differentially affects human natural killer cell subsets
Abstract
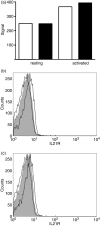
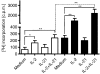
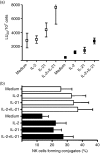
Interleukin-21 (IL-21) is a cytokine with pleiotropic effects on various cell types including dendritic cells, B cells, T cells and natural killer (NK) cells. To evaluate if IL-21 affects human NK cell subpopulations in a similar fashion, functional studies were performed on CD56(dim) and CD56(bright) NK cells, both bearing IL-21 receptors at identical densities. Stimulation with IL-21 strongly induced proliferation of CD56(bright) NK cells and cytotoxicity against K562 target cells was preferentially augmented in CD56(dim) NK cells. In contrast, stimulation with IL-2 and IL-21 alone or in combination failed to induce interferon-gamma and tumour necrosis factor-alpha production in the two NK cell subsets. Intracellular analysis of signal transducer and activator of transcription (STAT) proteins revealed that IL-21 by itself induces phosphorylation of STAT1 and STAT3 in CD56(dim) NK cells, and to an even higher degree in CD56(bright) NK cells. In this CD56(bright) NK cell population alone, IL-2 weakly phosphorylated STAT1 and STAT3, which was further increased when cells were treated with the combination of both cytokines. In contrast, STAT5 was strongly phosphorylated only in CD56(bright) NK cells by low-dose IL-2, while IL-21 did not affect STAT5 at all. In summary, we present data indicating that the NK-cell-directed cytokines IL-2 and IL-21 not only affect functions in NK cell subpopulations differently but can also act additively.
Figures
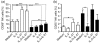

Similar articles
-
CD56bright natural killer (NK) cells: an important NK cell subset.Immunology. 2009 Apr;126(4):458-65. doi: 10.1111/j.1365-2567.2008.03027.x. Immunology. 2009. PMID: 19278419 Free PMC article. Review.
-
Human CD56bright and CD56dim natural killer cell subsets respond differentially to direct stimulation with Mycobacterium bovis bacillus Calmette-Guérin.Scand J Immunol. 2005 Dec;62(6):498-506. doi: 10.1111/j.1365-3083.2005.01692.x. Scand J Immunol. 2005. PMID: 16316416
-
Participation of the CD94 receptor complex in costimulation of human natural killer cells.J Immunol. 1998 Feb 15;160(4):1618-26. J Immunol. 1998. PMID: 9469418
-
Intracellular cytokine expression of peripheral blood natural killer cell subsets in women with recurrent spontaneous abortions and implantation failures.Fertil Steril. 2008 Jan;89(1):157-65. doi: 10.1016/j.fertnstert.2007.02.012. Epub 2007 May 7. Fertil Steril. 2008. PMID: 17482605
-
The biology of human natural killer-cell subsets.Trends Immunol. 2001 Nov;22(11):633-40. doi: 10.1016/s1471-4906(01)02060-9. Trends Immunol. 2001. PMID: 11698225 Review.
Cited by
-
Transcription of the activating receptor NKG2D in natural killer cells is regulated by STAT3 tyrosine phosphorylation.Blood. 2014 Jul 17;124(3):403-11. doi: 10.1182/blood-2013-05-499707. Epub 2014 Jun 2. Blood. 2014. PMID: 24891320 Free PMC article.
-
CD56bright natural killer (NK) cells: an important NK cell subset.Immunology. 2009 Apr;126(4):458-65. doi: 10.1111/j.1365-2567.2008.03027.x. Immunology. 2009. PMID: 19278419 Free PMC article. Review.
-
Murine models to study human NK cells in human solid tumors.Front Immunol. 2023 Jun 14;14:1209237. doi: 10.3389/fimmu.2023.1209237. eCollection 2023. Front Immunol. 2023. PMID: 37388731 Free PMC article. Review.
-
From bench to bedside: the past, present and future of IL-21 immunotherapy.Med Oncol. 2024 Jun 20;41(7):181. doi: 10.1007/s12032-024-02404-7. Med Oncol. 2024. PMID: 38900341 Review.
-
Interleukin-21 engineering enhances NK cell activity against glioblastoma via CEBPD.Cancer Cell. 2024 Aug 12;42(8):1450-1466.e11. doi: 10.1016/j.ccell.2024.07.007. Cancer Cell. 2024. PMID: 39137729 Free PMC article.
References
-
- Handa K, Suzuki R, Matsui H, Shimizu Y, Kumagai K. Natural killer (NK) cells as a responder to interleukin 2 (IL 2). II. IL-2-induced interferon gamma production. J Immunol. 1983;130:988–92. - PubMed
-
- Mehrotra PT, Donnelly RP, Wong S, et al. Production of IL-10 by human natural killer cells stimulated with IL-2 and/or IL-12. J Immunol. 1998;160:2637–44. - PubMed
-
- Fehniger TA, Shah MH, Turner MJ, et al. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol. 1999;162:4511–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

